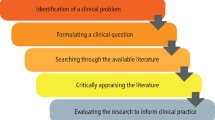
Conducting a Literature Review
- Literature Review
- Developing a Topic
- Planning Your Literature Review
- Developing a Search Strategy
- Managing Citations
- Critical Appraisal Tools
- Writing a Literature Review

Appraise Your Research Articles
The structure of a literature review should include the following :
- An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review,
- Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches entirely],
- An explanation of how each work is similar to and how it varies from the others,
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research.
The critical evaluation of each work should consider :
- Provenance -- what are the author's credentials? Are the author's arguments supported by evidence [e.g. primary historical material, case studies, narratives, statistics, recent scientific findings]?
- Methodology -- were the techniques used to identify, gather, and analyze the data appropriate to addressing the research problem? Was the sample size appropriate? Were the results effectively interpreted and reported?
- Objectivity -- is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
- Persuasiveness -- which of the author's theses are most convincing or least convincing?
- Value -- are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
Reviewing the Literature
While conducting a review of the literature, maximize the time you devote to writing this part of your paper by thinking broadly about what you should be looking for and evaluating. Review not just what the articles are saying, but how are they saying it.
Some questions to ask:
- How are they organizing their ideas?
- What methods have they used to study the problem?
- What theories have been used to explain, predict, or understand their research problem?
- What sources have they cited to support their conclusions?
- How have they used non-textual elements [e.g., charts, graphs, figures, etc.] to illustrate key points?
- When you begin to write your literature review section, you'll be glad you dug deeper into how the research was designed and constructed because it establishes a means for developing more substantial analysis and interpretation of the research problem.
Tools for Critical Appraisal
Now, that you have found articles based on your research question you can appraise the quality of those articles. These are resources you can use to appraise different study designs.
Centre for Evidence Based Medicine (Oxford)
University of Glasgow
"AFP uses the Strength-of-Recommendation Taxonomy (SORT), to label key recommendations in clinical review articles."
- SORT: Rating the Strength of Evidence American Family Physician and other family medicine journals use the Strength of Recommendation Taxonomy (SORT) system for rating bodies of evidence for key clinical recommendations.
- The Interprofessional Health Sciences Library
- 123 Metro Boulevard
- Nutley, NJ 07110
- [email protected]
- Student Services
- Parents and Families
- Career Center
- Web Accessibility
- Visiting Campus
- Public Safety
- Disability Support Services
- Campus Security Report
- Report a Problem
- Login to LibApps

Best Practice for Literature Searching
- Literature Search Best Practice
- What is literature searching?
- What are literature reviews?
- Hierarchies of evidence
- 1. Managing references
- 2. Defining your research question
- 3. Where to search
- 4. Search strategy
- 5. Screening results
- 6. Paper acquisition
- 7. Critical appraisal
- Further resources
- Training opportunities and videos
- Join FSTA student advisory board This link opens in a new window
- Chinese This link opens in a new window
- Italian This link opens in a new window
- Persian This link opens in a new window
- Portuguese This link opens in a new window
- Spanish This link opens in a new window
Deciding what to include in your review through critical appraisal
Once you have narrowed down your pool of results, it's time to begin critically appraising your articles. Using a checklist helps you scrutinise articles in a consistent, structured way.
Questions to consider include:
- Are the aims of the study clearly stated?
- Is the study design suitable for the aims?
- Are the measurements and methods used clearly described?
- Are the correct measurement tools used?
- Are the statistical methods described?
- Was the sample size adequate?
- Are the methods overall described in enough detail that you could replicate the study?
- Does the discussion overall reflect the results?
- Who funded this study?
- What are the specific limitations of what can be concluded from the study?
Working through the questions will help you identify the strengths and weakness of each article, and also identify points to draw on when you write about the literature.
- DOWNLOAD THE CRITICAL APPRAISAL CHECKLIST
Additional critical appraisal checklists

REFLECT provides a checklist for evaluating randomized control trials in livestock and food safety.

CASP provides checklists for critical appraisal of studies related to health.

JBI provides checklists for critical appraisal of studies related to health.
Documenting critical appraisal decisions
As you closely examine full articles, you will be making judgements about why to include or exclude each study from your review. Documenting your reasoning will help you reassure yourself and demonstrate to others that you have been systematic and unbiased in your appr aisal decisions.

Keeping track of what you have excluded, and why, will be very helpful if you must defend your work—for instance, if your literature review is part of a dissertation or thesis.

Pulling all the literature you will include in your review into a single chart is a good way to begin to synthesise the literature.
- DOWNLOAD THE FULL TEXT SCREENING CHART
Best practice!
BEST PRACTICE RECOMMENDATION : If you include any direct quotes in your chart (or in any notes) be sure to use quotation marks so that you don’t later mistake the words for your own.
BEST PRACTICE RECOMMENDATION: The more carefully you record each of the steps of your process, the more easily reproducible it will be. This is especially important for research abstracts and articles found in conference proceedings.
- << Previous: 6. Paper acquisition
- Next: Further resources >>
- Last Updated: May 17, 2024 5:48 PM
- URL: https://ifis.libguides.com/literature_search_best_practice
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 20 January 2009
How to critically appraise an article
- Jane M Young 1 &
- Michael J Solomon 2
Nature Clinical Practice Gastroenterology & Hepatology volume 6 , pages 82–91 ( 2009 ) Cite this article
52k Accesses
100 Citations
448 Altmetric
Metrics details
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article in order to assess the usefulness and validity of research findings. The most important components of a critical appraisal are an evaluation of the appropriateness of the study design for the research question and a careful assessment of the key methodological features of this design. Other factors that also should be considered include the suitability of the statistical methods used and their subsequent interpretation, potential conflicts of interest and the relevance of the research to one's own practice. This Review presents a 10-step guide to critical appraisal that aims to assist clinicians to identify the most relevant high-quality studies available to guide their clinical practice.
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article
Critical appraisal provides a basis for decisions on whether to use the results of a study in clinical practice
Different study designs are prone to various sources of systematic bias
Design-specific, critical-appraisal checklists are useful tools to help assess study quality
Assessments of other factors, including the importance of the research question, the appropriateness of statistical analysis, the legitimacy of conclusions and potential conflicts of interest are an important part of the critical appraisal process
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Making sense of the literature: an introduction to critical appraisal for the primary care practitioner
How to appraise the literature: basic principles for the busy clinician - part 2: systematic reviews and meta-analyses

How to appraise the literature: basic principles for the busy clinician - part 1: randomised controlled trials
Druss BG and Marcus SC (2005) Growth and decentralisation of the medical literature: implications for evidence-based medicine. J Med Libr Assoc 93 : 499–501
PubMed PubMed Central Google Scholar
Glasziou PP (2008) Information overload: what's behind it, what's beyond it? Med J Aust 189 : 84–85
PubMed Google Scholar
Last JE (Ed.; 2001) A Dictionary of Epidemiology (4th Edn). New York: Oxford University Press
Google Scholar
Sackett DL et al . (2000). Evidence-based Medicine. How to Practice and Teach EBM . London: Churchill Livingstone
Guyatt G and Rennie D (Eds; 2002). Users' Guides to the Medical Literature: a Manual for Evidence-based Clinical Practice . Chicago: American Medical Association
Greenhalgh T (2000) How to Read a Paper: the Basics of Evidence-based Medicine . London: Blackwell Medicine Books
MacAuley D (1994) READER: an acronym to aid critical reading by general practitioners. Br J Gen Pract 44 : 83–85
CAS PubMed PubMed Central Google Scholar
Hill A and Spittlehouse C (2001) What is critical appraisal. Evidence-based Medicine 3 : 1–8 [ http://www.evidence-based-medicine.co.uk ] (accessed 25 November 2008)
Public Health Resource Unit (2008) Critical Appraisal Skills Programme (CASP) . [ http://www.phru.nhs.uk/Pages/PHD/CASP.htm ] (accessed 8 August 2008)
National Health and Medical Research Council (2000) How to Review the Evidence: Systematic Identification and Review of the Scientific Literature . Canberra: NHMRC
Elwood JM (1998) Critical Appraisal of Epidemiological Studies and Clinical Trials (2nd Edn). Oxford: Oxford University Press
Agency for Healthcare Research and Quality (2002) Systems to rate the strength of scientific evidence? Evidence Report/Technology Assessment No 47, Publication No 02-E019 Rockville: Agency for Healthcare Research and Quality
Crombie IK (1996) The Pocket Guide to Critical Appraisal: a Handbook for Health Care Professionals . London: Blackwell Medicine Publishing Group
Heller RF et al . (2008) Critical appraisal for public health: a new checklist. Public Health 122 : 92–98
Article Google Scholar
MacAuley D et al . (1998) Randomised controlled trial of the READER method of critical appraisal in general practice. BMJ 316 : 1134–37
Article CAS Google Scholar
Parkes J et al . Teaching critical appraisal skills in health care settings (Review). Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No.: cd001270. 10.1002/14651858.cd001270
Mays N and Pope C (2000) Assessing quality in qualitative research. BMJ 320 : 50–52
Hawking SW (2003) On the Shoulders of Giants: the Great Works of Physics and Astronomy . Philadelphia, PN: Penguin
National Health and Medical Research Council (1999) A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines . Canberra: National Health and Medical Research Council
US Preventive Services Taskforce (1996) Guide to clinical preventive services (2nd Edn). Baltimore, MD: Williams & Wilkins
Solomon MJ and McLeod RS (1995) Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 118 : 456–467
Rothman KJ (2002) Epidemiology: an Introduction . Oxford: Oxford University Press
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: sources of bias in surgical studies. ANZ J Surg 73 : 504–506
Margitic SE et al . (1995) Lessons learned from a prospective meta-analysis. J Am Geriatr Soc 43 : 435–439
Shea B et al . (2001) Assessing the quality of reports of systematic reviews: the QUORUM statement compared to other tools. In Systematic Reviews in Health Care: Meta-analysis in Context 2nd Edition, 122–139 (Eds Egger M. et al .) London: BMJ Books
Chapter Google Scholar
Easterbrook PH et al . (1991) Publication bias in clinical research. Lancet 337 : 867–872
Begg CB and Berlin JA (1989) Publication bias and dissemination of clinical research. J Natl Cancer Inst 81 : 107–115
Moher D et al . (2000) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUORUM statement. Br J Surg 87 : 1448–1454
Shea BJ et al . (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 7 : 10 [10.1186/1471-2288-7-10]
Stroup DF et al . (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 : 2008–2012
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: evaluating surgical effectiveness. ANZ J Surg 73 : 507–510
Schulz KF (1995) Subverting randomization in controlled trials. JAMA 274 : 1456–1458
Schulz KF et al . (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273 : 408–412
Moher D et al . (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Medical Research Methodology 1 : 2 [ http://www.biomedcentral.com/ 1471-2288/1/2 ] (accessed 25 November 2008)
Rochon PA et al . (2005) Reader's guide to critical appraisal of cohort studies: 1. Role and design. BMJ 330 : 895–897
Mamdani M et al . (2005) Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330 : 960–962
Normand S et al . (2005) Reader's guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ 330 : 1021–1023
von Elm E et al . (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335 : 806–808
Sutton-Tyrrell K (1991) Assessing bias in case-control studies: proper selection of cases and controls. Stroke 22 : 938–942
Knottnerus J (2003) Assessment of the accuracy of diagnostic tests: the cross-sectional study. J Clin Epidemiol 56 : 1118–1128
Furukawa TA and Guyatt GH (2006) Sources of bias in diagnostic accuracy studies and the diagnostic process. CMAJ 174 : 481–482
Bossyut PM et al . (2003)The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138 : W1–W12
STARD statement (Standards for the Reporting of Diagnostic Accuracy Studies). [ http://www.stard-statement.org/ ] (accessed 10 September 2008)
Raftery J (1998) Economic evaluation: an introduction. BMJ 316 : 1013–1014
Palmer S et al . (1999) Economics notes: types of economic evaluation. BMJ 318 : 1349
Russ S et al . (1999) Barriers to participation in randomized controlled trials: a systematic review. J Clin Epidemiol 52 : 1143–1156
Tinmouth JM et al . (2004) Are claims of equivalency in digestive diseases trials supported by the evidence? Gastroentrology 126 : 1700–1710
Kaul S and Diamond GA (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 145 : 62–69
Piaggio G et al . (2006) Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295 : 1152–1160
Heritier SR et al . (2007) Inclusion of patients in clinical trial analysis: the intention to treat principle. In Interpreting and Reporting Clinical Trials: a Guide to the CONSORT Statement and the Principles of Randomized Controlled Trials , 92–98 (Eds Keech A. et al .) Strawberry Hills, NSW: Australian Medical Publishing Company
National Health and Medical Research Council (2007) National Statement on Ethical Conduct in Human Research 89–90 Canberra: NHMRC
Lo B et al . (2000) Conflict-of-interest policies for investigators in clinical trials. N Engl J Med 343 : 1616–1620
Kim SYH et al . (2004) Potential research participants' views regarding researcher and institutional financial conflicts of interests. J Med Ethics 30 : 73–79
Komesaroff PA and Kerridge IH (2002) Ethical issues concerning the relationships between medical practitioners and the pharmaceutical industry. Med J Aust 176 : 118–121
Little M (1999) Research, ethics and conflicts of interest. J Med Ethics 25 : 259–262
Lemmens T and Singer PA (1998) Bioethics for clinicians: 17. Conflict of interest in research, education and patient care. CMAJ 159 : 960–965
Download references
Author information
Authors and affiliations.
JM Young is an Associate Professor of Public Health and the Executive Director of the Surgical Outcomes Research Centre at the University of Sydney and Sydney South-West Area Health Service, Sydney,
Jane M Young
MJ Solomon is Head of the Surgical Outcomes Research Centre and Director of Colorectal Research at the University of Sydney and Sydney South-West Area Health Service, Sydney, Australia.,
Michael J Solomon
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jane M Young .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Young, J., Solomon, M. How to critically appraise an article. Nat Rev Gastroenterol Hepatol 6 , 82–91 (2009). https://doi.org/10.1038/ncpgasthep1331
Download citation
Received : 10 August 2008
Accepted : 03 November 2008
Published : 20 January 2009
Issue Date : February 2009
DOI : https://doi.org/10.1038/ncpgasthep1331
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Emergency physicians’ perceptions of critical appraisal skills: a qualitative study.
- Sumintra Wood
- Jacqueline Paulis
- Angela Chen
BMC Medical Education (2022)
An integrative review on individual determinants of enrolment in National Health Insurance Scheme among older adults in Ghana
- Anthony Kwame Morgan
- Anthony Acquah Mensah
BMC Primary Care (2022)
Autopsy findings of COVID-19 in children: a systematic review and meta-analysis
- Anju Khairwa
- Kana Ram Jat
Forensic Science, Medicine and Pathology (2022)
The use of a modified Delphi technique to develop a critical appraisal tool for clinical pharmacokinetic studies
- Alaa Bahaa Eldeen Soliman
- Shane Ashley Pawluk
- Ousama Rachid
International Journal of Clinical Pharmacy (2022)
Critical Appraisal: Analysis of a Prospective Comparative Study Published in IJS
- Ramakrishna Ramakrishna HK
- Swarnalatha MC
Indian Journal of Surgery (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- University of Texas Libraries
- UT Libraries
Systematic Reviews & Evidence Synthesis Methods
Critical appraisal.
- Types of Reviews
- Formulate Question
- Find Existing Reviews & Protocols
- Register a Protocol
- Searching Systematically
- Supplementary Searching
- Managing Results
- Deduplication
- Glossary of terms
- Librarian Support
- Video tutorials This link opens in a new window
- Systematic Review & Evidence Synthesis Boot Camp
Some reviews require a critical appraisal for each study that makes it through the screening process. This involves a risk of bias assessment and/or a quality assessment. The goal of these reviews is not just to find all of the studies, but to determine their methodological rigor, and therefore, their credibility.
"Critical appraisal is the balanced assessment of a piece of research, looking for its strengths and weaknesses and them coming to a balanced judgement about its trustworthiness and its suitability for use in a particular context." 1
It's important to consider the impact that poorly designed studies could have on your findings and to rule out inaccurate or biased work.
Selection of a valid critical appraisal tool, testing the tool with several of the selected studies, and involving two or more reviewers in the appraisal are good practices to follow.
1. Purssell E, McCrae N. How to Perform a Systematic Literature Review: A Guide for Healthcare Researchers, Practitioners and Students. 1st ed. Springer ; 2020.
Evaluation Tools
- The Appraisal of Guidelines for Research & Evaluation Instrument (AGREE II) The Appraisal of Guidelines for Research & Evaluation Instrument (AGREE II) was developed to address the issue of variability in the quality of practice guidelines.
- Centre for Evidence-Based Medicine (CEBM). Critical Appraisal Tools "contains useful tools and downloads for the critical appraisal of different types of medical evidence. Example appraisal sheets are provided together with several helpful examples."
- Critical Appraisal Skills Programme (CASP) Checklists Critical Appraisal checklists for many different study types
- Critical Review Form for Qualitative Studies Version 2, developed out of McMaster University
- Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. doi:10.1136/bmjopen-2016-011458
- Downs & Black Checklist for Assessing Studies Downs, S. H., & Black, N. (1998). The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. Journal of Epidemiology and Community Health (1979-), 52(6), 377–384.
- GRADE The Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group "has developed a common, sensible and transparent approach to grading quality (or certainty) of evidence and strength of recommendations."
- Grade Handbook Full handbook on the GRADE method for grading quality of evidence.
- MAGIC (Making GRADE the Irresistible choice) Clear succinct guidance in how to use GRADE
- Joanna Briggs Institute. Critical Appraisal Tools "JBI’s critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers." Includes checklists for 13 types of articles.
- Latitudes Network This is a searchable library of validity assessment tools for use in evidence syntheses. This website also provides access to training on the process of validity assessment.
- Mixed Methods Appraisal Tool A tool that can be used to appraise a mix of studies that are included in a systematic review - qualitative research, RCTs, non-randomized studies, quantitative studies, mixed methods studies.
- RoB 2 Tool Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I, Welch V (editors). Cochrane Methods. Cochrane Database of Systematic Reviews 2016, Issue 10 (Suppl 1). dx.doi.org/10.1002/14651858.CD201601.
- ROBINS-I Risk of Bias for non-randomized (observational) studies or cohorts of interventions Sterne J A, Hernán M A, Reeves B C, Savović J, Berkman N D, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ 2016; 355 :i4919 doi:10.1136/bmj.i4919
- Scottish Intercollegiate Guidelines Network. Critical Appraisal Notes and Checklists "Methodological assessment of studies selected as potential sources of evidence is based on a number of criteria that focus on those aspects of the study design that research has shown to have a significant effect on the risk of bias in the results reported and conclusions drawn. These criteria differ between study types, and a range of checklists is used to bring a degree of consistency to the assessment process."
- The TREND Statement (CDC) Des Jarlais DC, Lyles C, Crepaz N, and the TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am J Public Health. 2004;94:361-366.
- Assembling the Pieces of a Systematic Reviews, Chapter 8: Evaluating: Study Selection and Critical Appraisal.
- How to Perform a Systematic Literature Review, Chapter: Critical Appraisal: Assessing the Quality of Studies.
Other library guides
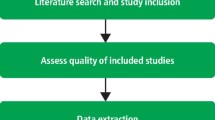
- Duke University Medical Center Library. Systematic Reviews: Assess for Quality and Bias
- UNC Health Sciences Library. Systematic Reviews: Assess Quality of Included Studies
- Last Updated: Jul 12, 2024 8:46 AM
- URL: https://guides.lib.utexas.edu/systematicreviews

- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
A guide to critical appraisal of evidence
Fineout-Overholt, Ellen PhD, RN, FNAP, FAAN
Ellen Fineout-Overholt is the Mary Coulter Dowdy Distinguished Professor of Nursing at the University of Texas at Tyler School of Nursing, Tyler, Tex.
The author has disclosed no financial relationships related to this article.
Critical appraisal is the assessment of research studies' worth to clinical practice. Critical appraisal—the heart of evidence-based practice—involves four phases: rapid critical appraisal, evaluation, synthesis, and recommendation. This article reviews each phase and provides examples, tips, and caveats to help evidence appraisers successfully determine what is known about a clinical issue. Patient outcomes are improved when clinicians apply a body of evidence to daily practice.
How do nurses assess the quality of clinical research? This article outlines a stepwise approach to critical appraisal of research studies' worth to clinical practice: rapid critical appraisal, evaluation, synthesis, and recommendation. When critical care nurses apply a body of valid, reliable, and applicable evidence to daily practice, patient outcomes are improved.

Critical care nurses can best explain the reasoning for their clinical actions when they understand the worth of the research supporting their practices. In c ritical appraisal , clinicians assess the worth of research studies to clinical practice. Given that achieving improved patient outcomes is the reason patients enter the healthcare system, nurses must be confident their care techniques will reliably achieve best outcomes.
Nurses must verify that the information supporting their clinical care is valid, reliable, and applicable. Validity of research refers to the quality of research methods used, or how good of a job researchers did conducting a study. Reliability of research means similar outcomes can be achieved when the care techniques of a study are replicated by clinicians. Applicability of research means it was conducted in a similar sample to the patients for whom the findings will be applied. These three criteria determine a study's worth in clinical practice.
Appraising the worth of research requires a standardized approach. This approach applies to both quantitative research (research that deals with counting things and comparing those counts) and qualitative research (research that describes experiences and perceptions). The word critique has a negative connotation. In the past, some clinicians were taught that studies with flaws should be discarded. Today, it is important to consider all valid and reliable research informative to what we understand as best practice. Therefore, the author developed the critical appraisal methodology that enables clinicians to determine quickly which evidence is worth keeping and which must be discarded because of poor validity, reliability, or applicability.
Evidence-based practice process
The evidence-based practice (EBP) process is a seven-step problem-solving approach that begins with data gathering (see Seven steps to EBP ). During daily practice, clinicians gather data supporting inquiry into a particular clinical issue (Step 0). The description is then framed as an answerable question (Step 1) using the PICOT question format ( P opulation of interest; I ssue of interest or intervention; C omparison to the intervention; desired O utcome; and T ime for the outcome to be achieved). 1 Consistently using the PICOT format helps ensure that all elements of the clinical issue are covered. Next, clinicians conduct a systematic search to gather data answering the PICOT question (Step 2). Using the PICOT framework, clinicians can systematically search multiple databases to find available studies to help determine the best practice to achieve the desired outcome for their patients. When the systematic search is completed, the work of critical appraisal begins (Step 3). The known group of valid and reliable studies that answers the PICOT question is called the body of evidence and is the foundation for the best practice implementation (Step 4). Next, clinicians evaluate integration of best evidence with clinical expertise and patient preferences and values to determine if the outcomes in the studies are realized in practice (Step 5). Because healthcare is a community of practice, it is important that experiences with evidence implementation be shared, whether the outcome is what was expected or not. This enables critical care nurses concerned with similar care issues to better understand what has been successful and what has not (Step 6).
Critical appraisal of evidence
The first phase of critical appraisal, rapid critical appraisal, begins with determining which studies will be kept in the body of evidence. All valid, reliable, and applicable studies on the topic should be included. This is accomplished using design-specific checklists with key markers of good research. When clinicians determine a study is one they want to keep (a “keeper” study) and that it belongs in the body of evidence, they move on to phase 2, evaluation. 2
In the evaluation phase, the keeper studies are put together in a table so that they can be compared as a body of evidence, rather than individual studies. This phase of critical appraisal helps clinicians identify what is already known about a clinical issue. In the third phase, synthesis, certain data that provide a snapshot of a particular aspect of the clinical issue are pulled out of the evaluation table to showcase what is known. These snapshots of information underpin clinicians' decision-making and lead to phase 4, recommendation. A recommendation is a specific statement based on the body of evidence indicating what should be done—best practice. Critical appraisal is not complete without a specific recommendation. Each of the phases is explained in more detail below.
Phase 1: Rapid critical appraisal . Rapid critical appraisal involves using two tools that help clinicians determine if a research study is worthy of keeping in the body of evidence. The first tool, General Appraisal Overview for All Studies (GAO), covers the basics of all research studies (see Elements of the General Appraisal Overview for All Studies ). Sometimes, clinicians find gaps in knowledge about certain elements of research studies (for example, sampling or statistics) and need to review some content. Conducting an internet search for resources that explain how to read a research paper, such as an instructional video or step-by-step guide, can be helpful. Finding basic definitions of research methods often helps resolve identified gaps.
To accomplish the GAO, it is best to begin with finding out why the study was conducted and how it answers the PICOT question (for example, does it provide information critical care nurses want to know from the literature). If the study purpose helps answer the PICOT question, then the type of study design is evaluated. The study design is compared with the hierarchy of evidence for the type of PICOT question. The higher the design falls within the hierarchy or levels of evidence, the more confidence nurses can have in its finding, if the study was conducted well. 3,4 Next, find out what the researchers wanted to learn from their study. These are called the research questions or hypotheses. Research questions are just what they imply; insufficient information from theories or the literature are available to guide an educated guess, so a question is asked. Hypotheses are reasonable expectations guided by understanding from theory and other research that predicts what will be found when the research is conducted. The research questions or hypotheses provide the purpose of the study.
Next, the sample size is evaluated. Expectations of sample size are present for every study design. As an example, consider as a rule that quantitative study designs operate best when there is a sample size large enough to establish that relationships do not exist by chance. In general, the more participants in a study, the more confidence in the findings. Qualitative designs operate best with fewer people in the sample because these designs represent a deeper dive into the understanding or experience of each person in the study. 5 It is always important to describe the sample, as clinicians need to know if the study sample resembles their patients. It is equally important to identify the major variables in the study and how they are defined because this helps clinicians best understand what the study is about.
The final step in the GAO is to consider the analyses that answer the study research questions or confirm the study hypothesis. This is another opportunity for clinicians to learn, as learning about statistics in healthcare education has traditionally focused on conducting statistical tests as opposed to interpreting statistical tests. Understanding what the statistics indicate about the study findings is an imperative of critical appraisal of quantitative evidence.
The second tool is one of the variety of rapid critical appraisal checklists that speak to validity, reliability, and applicability of specific study designs, which are available at varying locations (see Critical appraisal resources ). When choosing a checklist to implement with a group of critical care nurses, it is important to verify that the checklist is complete and simple to use. Be sure to check that the checklist has answers to three key questions. The first question is: Are the results of the study valid? Related subquestions should help nurses discern if certain markers of good research design are present within the study. For example, identifying that study participants were randomly assigned to study groups is an essential marker of good research for a randomized controlled trial. Checking these essential markers helps clinicians quickly review a study to check off these important requirements. Clinical judgment is required when the study lacks any of the identified quality markers. Clinicians must discern whether the absence of any of the essential markers negates the usefulness of the study findings. 6-9

The second question is: What are the study results? This is answered by reviewing whether the study found what it was expecting to and if those findings were meaningful to clinical practice. Basic knowledge of how to interpret statistics is important for understanding quantitative studies, and basic knowledge of qualitative analysis greatly facilitates understanding those results. 6-9
The third question is: Are the results applicable to my patients? Answering this question involves consideration of the feasibility of implementing the study findings into the clinicians' environment as well as any contraindication within the clinicians' patient populations. Consider issues such as organizational politics, financial feasibility, and patient preferences. 6-9
When these questions have been answered, clinicians must decide about whether to keep the particular study in the body of evidence. Once the final group of keeper studies is identified, clinicians are ready to move into the phase of critical appraisal. 6-9
Phase 2: Evaluation . The goal of evaluation is to determine how studies within the body of evidence agree or disagree by identifying common patterns of information across studies. For example, an evaluator may compare whether the same intervention is used or if the outcomes are measured in the same way across all studies. A useful tool to help clinicians accomplish this is an evaluation table. This table serves two purposes: first, it enables clinicians to extract data from the studies and place the information in one table for easy comparison with other studies; and second, it eliminates the need for further searching through piles of periodicals for the information. (See Bonus Content: Evaluation table headings .) Although the information for each of the columns may not be what clinicians consider as part of their daily work, the information is important for them to understand about the body of evidence so that they can explain the patterns of agreement or disagreement they identify across studies. Further, the in-depth understanding of the body of evidence from the evaluation table helps with discussing the relevant clinical issue to facilitate best practice. Their discussion comes from a place of knowledge and experience, which affords the most confidence. The patterns and in-depth understanding are what lead to the synthesis phase of critical appraisal.
The key to a successful evaluation table is simplicity. Entering data into the table in a simple, consistent manner offers more opportunity for comparing studies. 6-9 For example, using abbreviations versus complete sentences in all columns except the final one allows for ease of comparison. An example might be the dependent variable of depression defined as “feelings of severe despondency and dejection” in one study and as “feeling sad and lonely” in another study. 10 Because these are two different definitions, they need to be different dependent variables. Clinicians must use their clinical judgment to discern that these different dependent variables require different names and abbreviations and how these further their comparison across studies.

Sample and theoretical or conceptual underpinnings are important to understanding how studies compare. Similar samples and settings across studies increase agreement. Several studies with the same conceptual framework increase the likelihood of common independent variables and dependent variables. The findings of a study are dependent on the analyses conducted. That is why an analysis column is dedicated to recording the kind of analysis used (for example, the name of the statistical analyses for quantitative studies). Only statistics that help answer the clinical question belong in this column. The findings column must have a result for each of the analyses listed; however, in the actual results, not in words. For example, a clinician lists a t -test as a statistic in the analysis column, so a t -value should reflect whether the groups are different as well as probability ( P -value or confidence interval) that reflects statistical significance. The explanation for these results would go in the last column that describes worth of the research to practice. This column is much more flexible and contains other information such as the level of evidence, the studies' strengths and limitations, any caveats about the methodology, or other aspects of the study that would be helpful to its use in practice. The final piece of information in this column is a recommendation for how this study would be used in practice. Each of the studies in the body of evidence that addresses the clinical question is placed in one evaluation table to facilitate the ease of comparing across the studies. This comparison sets the stage for synthesis.
Phase 3: Synthesis . In the synthesis phase, clinicians pull out key information from the evaluation table to produce a snapshot of the body of evidence. A table also is used here to feature what is known and help all those viewing the synthesis table to come to the same conclusion. A hypothetical example table included here demonstrates that a music therapy intervention is effective in reducing the outcome of oxygen saturation (SaO 2 ) in six of the eight studies in the body of evidence that evaluated that outcome (see Sample synthesis table: Impact on outcomes ). Simply using arrows to indicate effect offers readers a collective view of the agreement across studies that prompts action. Action may be to change practice, affirm current practice, or conduct research to strengthen the body of evidence by collaborating with nurse scientists.
When synthesizing evidence, there are at least two recommended synthesis tables, including the level-of-evidence table and the impact-on-outcomes table for quantitative questions, such as therapy or relevant themes table for “meaning” questions about human experience. (See Bonus Content: Level of evidence for intervention studies: Synthesis of type .) The sample synthesis table also demonstrates that a final column labeled synthesis indicates agreement across the studies. Of the three outcomes, the most reliable for clinicians to see with music therapy is SaO 2 , with positive results in six out of eight studies. The second most reliable outcome would be reducing increased respiratory rate (RR). Parental engagement has the least support as a reliable outcome, with only two of five studies showing positive results. Synthesis tables make the recommendation clear to all those who are involved in caring for that patient population. Although the two synthesis tables mentioned are a great start, the evidence may require more synthesis tables to adequately explain what is known. These tables are the foundation that supports clinically meaningful recommendations.
Phase 4: Recommendation . Recommendations are definitive statements based on what is known from the body of evidence. For example, with an intervention question, clinicians should be able to discern from the evidence if they will reliably get the desired outcome when they deliver the intervention as it was in the studies. In the sample synthesis table, the recommendation would be to implement the music therapy intervention across all settings with the population, and measure SaO 2 and RR, with the expectation that both would be optimally improved with the intervention. When the synthesis demonstrates that studies consistently verify an outcome occurs as a result of an intervention, however that intervention is not currently practiced, care is not best practice. Therefore, a firm recommendation to deliver the intervention and measure the appropriate outcomes must be made, which concludes critical appraisal of the evidence.
A recommendation that is off limits is conducting more research, as this is not the focus of clinicians' critical appraisal. In the case of insufficient evidence to make a recommendation for practice change, the recommendation would be to continue current practice and monitor outcomes and processes until there are more reliable studies to be added to the body of evidence. Researchers who use the critical appraisal process may indeed identify gaps in knowledge, research methods, or analyses, for example, that they then recommend studies that would fill in the identified gaps. In this way, clinicians and nurse scientists work together to build relevant, efficient bodies of evidence that guide clinical practice.
Evidence into action
Critical appraisal helps clinicians understand the literature so they can implement it. Critical care nurses have a professional and ethical responsibility to make sure their care is based on a solid foundation of available evidence that is carefully appraised using the phases outlined here. Critical appraisal allows for decision-making based on evidence that demonstrates reliable outcomes. Any other approach to the literature is likely haphazard and may lead to misguided care and unreliable outcomes. 11 Evidence translated into practice should have the desired outcomes and their measurement defined from the body of evidence. It is also imperative that all critical care nurses carefully monitor care delivery outcomes to establish that best outcomes are sustained. With the EBP paradigm as the basis for decision-making and the EBP process as the basis for addressing clinical issues, critical care nurses can improve patient, provider, and system outcomes by providing best care.
Seven steps to EBP
Step 0–A spirit of inquiry to notice internal data that indicate an opportunity for positive change.
Step 1– Ask a clinical question using the PICOT question format.
Step 2–Conduct a systematic search to find out what is already known about a clinical issue.
Step 3–Conduct a critical appraisal (rapid critical appraisal, evaluation, synthesis, and recommendation).
Step 4–Implement best practices by blending external evidence with clinician expertise and patient preferences and values.
Step 5–Evaluate evidence implementation to see if study outcomes happened in practice and if the implementation went well.
Step 6–Share project results, good or bad, with others in healthcare.
Adapted from: Steps of the evidence-based practice (EBP) process leading to high-quality healthcare and best patient outcomes. © Melnyk & Fineout-Overholt, 2017. Used with permission.
Critical appraisal resources
- The Joanna Briggs Institute http://joannabriggs.org/research/critical-appraisal-tools.html
- Critical Appraisal Skills Programme (CASP) www.casp-uk.net/casp-tools-checklists
- Center for Evidence-Based Medicine www.cebm.net/critical-appraisal
- Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing and Healthcare: A Guide to Best Practice . 3rd ed. Philadelphia, PA: Wolters Kluwer; 2015.
A full set of critical appraisal checklists are available in the appendices.
Bonus content!
This article includes supplementary online-exclusive material. Visit the online version of this article at www.nursingcriticalcare.com to access this content.
critical appraisal; decision-making; evaluation of research; evidence-based practice; synthesis
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Determining the level of evidence: experimental research appraisal, nurse-driven protocols, evidence-based practice for red blood cell transfusions, recognizing and preventing drug diversion, pulmonary embolism: prevention, recognition, and treatment.
- Mayo Clinic Libraries
- Systematic Reviews
- Critical Appraisal by Study Design
Systematic Reviews: Critical Appraisal by Study Design
- Knowledge Synthesis Comparison
- Knowledge Synthesis Decision Tree
- Standards & Reporting Results
- Materials in the Mayo Clinic Libraries
- Training Resources
- Review Teams
- Develop & Refine Your Research Question
- Develop a Timeline
- Project Management
- Communication
- PRISMA-P Checklist
- Eligibility Criteria
- Register your Protocol
- Other Resources
- Other Screening Tools
- Grey Literature Searching
- Citation Searching
- Data Extraction Tools
- Minimize Bias
- Synthesis & Meta-Analysis
- Publishing your Systematic Review
Tools for Critical Appraisal of Studies

“The purpose of critical appraisal is to determine the scientific merit of a research report and its applicability to clinical decision making.” 1 Conducting a critical appraisal of a study is imperative to any well executed evidence review, but the process can be time consuming and difficult. 2 The critical appraisal process requires “a methodological approach coupled with the right tools and skills to match these methods is essential for finding meaningful results.” 3 In short, it is a method of differentiating good research from bad research.
Critical Appraisal by Study Design (featured tools)
- Non-RCTs or Observational Studies
- Diagnostic Accuracy
- Animal Studies
- Qualitative Research
- Tool Repository
- AMSTAR 2 The original AMSTAR was developed to assess the risk of bias in systematic reviews that included only randomized controlled trials. AMSTAR 2 was published in 2017 and allows researchers to “identify high quality systematic reviews, including those based on non-randomised studies of healthcare interventions.” 4 more... less... AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews)
- ROBIS ROBIS is a tool designed specifically to assess the risk of bias in systematic reviews. “The tool is completed in three phases: (1) assess relevance(optional), (2) identify concerns with the review process, and (3) judge risk of bias in the review. Signaling questions are included to help assess specific concerns about potential biases with the review.” 5 more... less... ROBIS (Risk of Bias in Systematic Reviews)
- BMJ Framework for Assessing Systematic Reviews This framework provides a checklist that is used to evaluate the quality of a systematic review.
- CASP Checklist for Systematic Reviews This CASP checklist is not a scoring system, but rather a method of appraising systematic reviews by considering: 1. Are the results of the study valid? 2. What are the results? 3. Will the results help locally? more... less... CASP (Critical Appraisal Skills Programme)
- CEBM Systematic Reviews Critical Appraisal Sheet The CEBM’s critical appraisal sheets are designed to help you appraise the reliability, importance, and applicability of clinical evidence. more... less... CEBM (Centre for Evidence-Based Medicine)
- JBI Critical Appraisal Tools, Checklist for Systematic Reviews JBI Critical Appraisal Tools help you assess the methodological quality of a study and to determine the extent to which study has addressed the possibility of bias in its design, conduct and analysis.
- NHLBI Study Quality Assessment of Systematic Reviews and Meta-Analyses The NHLBI’s quality assessment tools were designed to assist reviewers in focusing on concepts that are key for critical appraisal of the internal validity of a study. more... less... NHLBI (National Heart, Lung, and Blood Institute)
- RoB 2 RoB 2 “provides a framework for assessing the risk of bias in a single estimate of an intervention effect reported from a randomized trial,” rather than the entire trial. 6 more... less... RoB 2 (revised tool to assess Risk of Bias in randomized trials)
- CASP Randomised Controlled Trials Checklist This CASP checklist considers various aspects of an RCT that require critical appraisal: 1. Is the basic study design valid for a randomized controlled trial? 2. Was the study methodologically sound? 3. What are the results? 4. Will the results help locally? more... less... CASP (Critical Appraisal Skills Programme)
- CONSORT Statement The CONSORT checklist includes 25 items to determine the quality of randomized controlled trials. “Critical appraisal of the quality of clinical trials is possible only if the design, conduct, and analysis of RCTs are thoroughly and accurately described in the report.” 7 more... less... CONSORT (Consolidated Standards of Reporting Trials)
- NHLBI Study Quality Assessment of Controlled Intervention Studies The NHLBI’s quality assessment tools were designed to assist reviewers in focusing on concepts that are key for critical appraisal of the internal validity of a study. more... less... NHLBI (National Heart, Lung, and Blood Institute)
- JBI Critical Appraisal Tools Checklist for Randomized Controlled Trials JBI Critical Appraisal Tools help you assess the methodological quality of a study and to determine the extent to which study has addressed the possibility of bias in its design, conduct and analysis.
- ROBINS-I ROBINS-I is a “tool for evaluating risk of bias in estimates of the comparative effectiveness… of interventions from studies that did not use randomization to allocate units… to comparison groups.” 8 more... less... ROBINS-I (Risk Of Bias in Non-randomized Studies – of Interventions)
- NOS This tool is used primarily to evaluate and appraise case-control or cohort studies. more... less... NOS (Newcastle-Ottawa Scale)
- AXIS Cross-sectional studies are frequently used as an evidence base for diagnostic testing, risk factors for disease, and prevalence studies. “The AXIS tool focuses mainly on the presented [study] methods and results.” 9 more... less... AXIS (Appraisal tool for Cross-Sectional Studies)
- NHLBI Study Quality Assessment Tools for Non-Randomized Studies The NHLBI’s quality assessment tools were designed to assist reviewers in focusing on concepts that are key for critical appraisal of the internal validity of a study. • Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies • Quality Assessment of Case-Control Studies • Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group • Quality Assessment Tool for Case Series Studies more... less... NHLBI (National Heart, Lung, and Blood Institute)
- Case Series Studies Quality Appraisal Checklist Developed by the Institute of Health Economics (Canada), the checklist is comprised of 20 questions to assess “the robustness of the evidence of uncontrolled, [case series] studies.” 10
- Methodological Quality and Synthesis of Case Series and Case Reports In this paper, Dr. Murad and colleagues “present a framework for appraisal, synthesis and application of evidence derived from case reports and case series.” 11
- MINORS The MINORS instrument contains 12 items and was developed for evaluating the quality of observational or non-randomized studies. 12 This tool may be of particular interest to researchers who would like to critically appraise surgical studies. more... less... MINORS (Methodological Index for Non-Randomized Studies)
- JBI Critical Appraisal Tools for Non-Randomized Trials JBI Critical Appraisal Tools help you assess the methodological quality of a study and to determine the extent to which study has addressed the possibility of bias in its design, conduct and analysis. • Checklist for Analytical Cross Sectional Studies • Checklist for Case Control Studies • Checklist for Case Reports • Checklist for Case Series • Checklist for Cohort Studies
- QUADAS-2 The QUADAS-2 tool “is designed to assess the quality of primary diagnostic accuracy studies… [it] consists of 4 key domains that discuss patient selection, index test, reference standard, and flow of patients through the study and timing of the index tests and reference standard.” 13 more... less... QUADAS-2 (a revised tool for the Quality Assessment of Diagnostic Accuracy Studies)
- JBI Critical Appraisal Tools Checklist for Diagnostic Test Accuracy Studies JBI Critical Appraisal Tools help you assess the methodological quality of a study and to determine the extent to which study has addressed the possibility of bias in its design, conduct and analysis.
- STARD 2015 The authors of the standards note that “[e]ssential elements of [diagnostic accuracy] study methods are often poorly described and sometimes completely omitted, making both critical appraisal and replication difficult, if not impossible.”10 The Standards for the Reporting of Diagnostic Accuracy Studies was developed “to help… improve completeness and transparency in reporting of diagnostic accuracy studies.” 14 more... less... STARD 2015 (Standards for the Reporting of Diagnostic Accuracy Studies)
- CASP Diagnostic Study Checklist This CASP checklist considers various aspects of diagnostic test studies including: 1. Are the results of the study valid? 2. What were the results? 3. Will the results help locally? more... less... CASP (Critical Appraisal Skills Programme)
- CEBM Diagnostic Critical Appraisal Sheet The CEBM’s critical appraisal sheets are designed to help you appraise the reliability, importance, and applicability of clinical evidence. more... less... CEBM (Centre for Evidence-Based Medicine)
- SYRCLE’s RoB “[I]mplementation of [SYRCLE’s RoB tool] will facilitate and improve critical appraisal of evidence from animal studies. This may… enhance the efficiency of translating animal research into clinical practice and increase awareness of the necessity of improving the methodological quality of animal studies.” 15 more... less... SYRCLE’s RoB (SYstematic Review Center for Laboratory animal Experimentation’s Risk of Bias)
- ARRIVE 2.0 “The [ARRIVE 2.0] guidelines are a checklist of information to include in a manuscript to ensure that publications [on in vivo animal studies] contain enough information to add to the knowledge base.” 16 more... less... ARRIVE 2.0 (Animal Research: Reporting of In Vivo Experiments)
- Critical Appraisal of Studies Using Laboratory Animal Models This article provides “an approach to critically appraising papers based on the results of laboratory animal experiments,” and discusses various “bias domains” in the literature that critical appraisal can identify. 17
- CEBM Critical Appraisal of Qualitative Studies Sheet The CEBM’s critical appraisal sheets are designed to help you appraise the reliability, importance and applicability of clinical evidence. more... less... CEBM (Centre for Evidence-Based Medicine)
- CASP Qualitative Studies Checklist This CASP checklist considers various aspects of qualitative research studies including: 1. Are the results of the study valid? 2. What were the results? 3. Will the results help locally? more... less... CASP (Critical Appraisal Skills Programme)
- Quality Assessment and Risk of Bias Tool Repository Created by librarians at Duke University, this extensive listing contains over 100 commonly used risk of bias tools that may be sorted by study type.
- Latitudes Network A library of risk of bias tools for use in evidence syntheses that provides selection help and training videos.
References & Recommended Reading
1. Kolaski, K., Logan, L. R., & Ioannidis, J. P. (2024). Guidance to best tools and practices for systematic reviews . British Journal of Pharmacology , 181 (1), 180-210
2. Portney LG. Foundations of clinical research : applications to evidence-based practice. Fourth edition. ed. Philadelphia: F A Davis; 2020.
3. Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ (Clinical research ed). 1991;302(6785):1136-1140.
4. Singh S. Critical appraisal skills programme. Journal of Pharmacology and Pharmacotherapeutics. 2013;4(1):76-77.
5. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). 2017;358:j4008.
6. Whiting P, Savovic J, Higgins JPT, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of clinical epidemiology. 2016;69:225-234.
7. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366:l4898.
8. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. Journal of clinical epidemiology. 2010;63(8):e1-37.
9. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical research ed). 2016;355:i4919.
10. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ open. 2016;6(12):e011458.
11. Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. Journal of clinical epidemiology. 2016;69:199-207.e192.
12. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ evidence-based medicine. 2018;23(2):60-63.
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ journal of surgery. 2003;73(9):712-716.
14. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529-536.
15. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ (Clinical research ed). 2015;351:h5527.
16. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC medical research methodology. 2014;14:43.
17. Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS biology. 2020;18(7):e3000411.
18. O'Connor AM, Sargeant JM. Critical appraisal of studies using laboratory animal models. ILAR journal. 2014;55(3):405-417.
- << Previous: Minimize Bias
- Next: GRADE >>
- Last Updated: Jul 8, 2024 12:54 PM
- URL: https://libraryguides.mayo.edu/systematicreviewprocess
Please enter both an email address and a password.
Account login
- Show/Hide Password Show password Hide password
- Reset Password
Need to reset your password? Enter the email address which you used to register on this site (or your membership/contact number) and we'll email you a link to reset it. You must complete the process within 2hrs of receiving the link.
We've sent you an email.
An email has been sent to Simply follow the link provided in the email to reset your password. If you can't find the email please check your junk or spam folder and add [email protected] to your address book.
- About RCS England

- Dissecting the literature: the importance of critical appraisal
08 Dec 2017
Kirsty Morrison
This post was updated in 2023.
Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context.
Amanda Burls, What is Critical Appraisal?
Why is critical appraisal needed?
Literature searches using databases like Medline or EMBASE often result in an overwhelming volume of results which can vary in quality. Similarly, those who browse medical literature for the purposes of CPD or in response to a clinical query will know that there are vast amounts of content available. Critical appraisal helps to reduce the burden and allow you to focus on articles that are relevant to the research question, and that can reliably support or refute its claims with high-quality evidence, or identify high-level research relevant to your practice.

Critical appraisal allows us to:
- reduce information overload by eliminating irrelevant or weak studies
- identify the most relevant papers
- distinguish evidence from opinion, assumptions, misreporting, and belief
- assess the validity of the study
- assess the usefulness and clinical applicability of the study
- recognise any potential for bias.
Critical appraisal helps to separate what is significant from what is not. One way we use critical appraisal in the Library is to prioritise the most clinically relevant content for our Current Awareness Updates .
How to critically appraise a paper
There are some general rules to help you, including a range of checklists highlighted at the end of this blog. Some key questions to consider when critically appraising a paper:
- Is the study question relevant to my field?
- Does the study add anything new to the evidence in my field?
- What type of research question is being asked? A well-developed research question usually identifies three components: the group or population of patients, the studied parameter (e.g. a therapy or clinical intervention) and outcomes of interest.
- Was the study design appropriate for the research question? You can learn more about different study types and the hierarchy of evidence here .
- Did the methodology address important potential sources of bias? Bias can be attributed to chance (e.g. random error) or to the study methods (systematic bias).
- Was the study performed according to the original protocol? Deviations from the planned protocol can affect the validity or relevance of a study, e.g. a decrease in the studied population over the course of a randomised controlled trial .
- Does the study test a stated hypothesis? Is there a clear statement of what the investigators expect the study to find which can be tested, and confirmed or refuted.
- Were the statistical analyses performed correctly? The approach to dealing with missing data, and the statistical techniques that have been applied should be specified. Original data should be presented clearly so that readers can check the statistical accuracy of the paper.
- Do the data justify the conclusions? Watch out for definite conclusions based on statistically insignificant results, generalised findings from a small sample size, and statistically significant associations being misinterpreted to imply a cause and effect.
- Are there any conflicts of interest? Who has funded the study and can we trust their objectivity? Do the authors have any potential conflicts of interest, and have these been declared?
And an important consideration for surgeons:
- Will the results help me manage my patients?
At the end of the appraisal process you should have a better appreciation of how strong the evidence is, and ultimately whether or not you should apply it to your patients.
Further resources:
- How to Read a Paper by Trisha Greenhalgh
- The Doctor’s Guide to Critical Appraisal by Narinder Kaur Gosall
- CASP checklists
- CEBM Critical Appraisal Tools
- Critical Appraisal: a checklist
- Critical Appraisal of a Journal Article (PDF)
- Introduction to...Critical appraisal of literature
- Reporting guidelines for the main study types
Kirsty Morrison, Information Specialist
Share this page:
- Library Blog
Literature review methods
Critical appraisal, assess for quality.
Critical appraisal refers to the process of judging the validity and quality of a research paper. Because your review will be a synthesis of the research conducted by others, it is important to consider major points about the studies you include such as:
- Is the study valid?
- What are the results, and are they significant or trustworthy?
- Are the results applicable to your research question?
Critical appraisal tools
Several checklists or tools are available to help you assess the quality of studies to be included in your review.
- AACODS checklist for appraising grey literature
- CASP includes checklists for systematic reviews, RCTs, qualitative studies etc.
- Centre for Evidence Based Medicine appraisal worksheets to appraise the reliability, importance and applicability of clinical evidence
- Joanna Briggs Institute critical appraisal tools to assess the trustworthiness, relevance and results of published papers
- RoBiS tool for assessing the risk of bias in systematic reviews (rather than in primary studies)
- RoB 2 Cochrane risk-of-bias tool for randomized trials
Additional resources
- Greenhalgh, T. (2019). How to read a paper: the basics of evidence-based medicine and healthcare (6th ed). Wiley.
- << Previous: Screening
- Next: Synthesis analysis >>
Contact the library
- Ask the library
- Book time for search help
- Suggest an acquisition
- 036-10 10 10
- Follow us on Instagram
- Follow us on Facebook
Visiting address
University library Jönköping University campus, building C Gjuterigatan 5 553 18 Jönköping
- Delivery addresses
Opening hours
- Mondays 8 – 20
- Tuesdays 8 – 20
- Wednesdays 8 – 20
- Thursdays 8 – 20
- Fridays 8 – 18
- Saturdays 11 – 15
- Sundays Closed
See more opening hours .
- Search Menu
- Sign in through your institution
- Advance Articles
- Specialty Certificate Examination Cases
- Cover Archive
- Virtual Issues
- Trending Articles
- Author Guidelines
- Reviewer Guidelines
- Submission Site
- Open Access Options
- Reasons to Publish
- Self-Archiving Policy
- About Clinical and Experimental Dermatology
- About British Association of Dermatologists
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Discussion and conclusions, learning points, funding sources, data availability, ethics statement, cpd questions, instructions for answering questions.
- < Previous
How to critically appraise a systematic review: an aide for the reader and reviewer
Conflicts of interest H.W. founded the Cochrane Skin Group in 1987 and was coordinator editor until 2018. The other authors declare they have no conflicts of interest.
- Article contents
- Figures & tables
- Supplementary Data
John Frewen, Marianne de Brito, Anjali Pathak, Richard Barlow, Hywel C Williams, How to critically appraise a systematic review: an aide for the reader and reviewer, Clinical and Experimental Dermatology , Volume 48, Issue 8, August 2023, Pages 854–859, https://doi.org/10.1093/ced/llad141
- Permissions Icon Permissions
The number of published systematic reviews has soared rapidly in recent years. Sadly, the quality of most systematic reviews in dermatology is substandard. With the continued increase in exposure to systematic reviews, and their potential to influence clinical practice, we sought to describe a sequence of useful tips for the busy clinician reader to determine study quality and clinical utility. Important factors to consider when assessing systematic reviews include: determining the motivation to performing the study, establishing if the study protocol was prepublished, assessing quality of reporting using the PRISMA checklist, assessing study quality using the AMSTAR 2 critical appraisal checklist, assessing for evidence of spin, and summarizing the main strengths and limitations of the study to determine if it could change clinical practice. Having a set of heuristics to consider when reading systematic reviews serves to save time, enabling assessment of quality in a structured way, and come to a prompt conclusion of the merits of a review article in order to inform the care of dermatology patients.
A systematic review aims to systematically and transparently summarize the available data on a defined clinical question, via a rigorous search for studies, a critique of the quality of included studies and a qualitative and/or quantitative synthesis. 1 Systematic reviews are at the top of the pyramid in most evidence hierarchies for informing evidence-based healthcare as they are considered of greater validity and clinical applicability than those study types lower down, such as case series or individual trials. 2
A good systematic review should provide an unbiased overview of studies to inform clinical practice. Systematic reviews can reconcile apparently conflicting results, add precision to estimating smaller treatment effects, highlight the evidence’s limitations and biases and identify research gaps. Guidelines are available to assist systematic reviewers to transparently report why the review was done, the authors’ methods and findings via the PRISMA checklist. 3
The sharp rise in systematic review publications over time raises concern that the majority are unnecessary, misleading and/or conflicted. 4 A review of dermatology systematic reviews noted that 93% failed to report at least one PRISMA checklist item. 5 Another review of a random sample of 140/732 dermatology systematic reviews in 2017 found 90% were low quality. 6 Some improvements have occurred: reporting standards compliance has improved slightly (between 2013 and 2017), 5 and several leading dermatology journals including the British Journal of Dermatology have changed editorial policies, mandating authors to preregister review protocols.
Given the surge in poor-quality systematic review publications, we sought to describe a checklist of seven practical tips from the authors’ collective experience of writing and critically appraising systematic reviews, hoping that they will assist busy clinicians to critically appraise systematic reviews both as manuscript reviewers and as readers and research users.
Read the abstract to develop a sense of the subject.
What was the motivation for completing the review?
Has the review protocol been published and have changes been made to it.
Review the reporting quality .
Review the quality of the article and the depth of the review question.
Consider the authors’ interpretation and assess for spin .
Summarize and come to a position .
Read the abstract to develop a sense of the subject
From the abstract, use the PICO (population, intervention, comparator and outcome) framework to establish if the subject, intervention and outcomes are relevant to clinical practice. Is the review question clear and appropriate?
Inspect the authors’ conflicts of interest and funding sources. Self-disclosed financial conflicts are often insufficiently described or not declared at all. 7 If you suspect conflicts for authors with no stated conflicts, briefly searching the senior authors’ names on PubMed, or the Open Payments website (for US authors) may reveal hidden conflicts. 8 Is the motivation for the systematic review justified in the introduction? Can new insights be formed by combining studies? If the systematic review is an update, what new available data justifies this? Search for similar recent systematic reviews (which may have been omitted intentionally). Is it a redundant duplicate review that adds little new useful information? 9 Has the author recently published reviews on similar subjects? Salami publications refer to authors chopping up a topic into smaller pieces to obtain maximum publications. 10
Search PROSPERO for publication of the review protocol. 11 A prepublished review protocol in a publicly accessible site offers reassurance that the systematic review followed a clear plan with prespecified PICO elements. Put bluntly, it reduces authors’ opportunity for deception by selective analysis and highlighting of results that are more likely to get published. If a protocol is found, assess deviation from this protocol and justification, if present. Protocol registration allows improved PRISMA reporting. 12 A registered protocol with reporting of deviations allows the reader to judge whether any modifications are justified, for example adjusting for unexpected challenges during analysis. 10
Review the reporting quality
Look for supplementary material detailing the PRISMA checklist. Commonly under-reported PRISMA items include protocol and registration, risk of bias across studies, risk of bias in individual studies, the data collection process and review objectives. 5 Adequate reporting quality using PRISMA does not necessarily indicate the review is clinically useful; however, it allows the reader to assess the study’s utility (see Table 1 ). Additional assessments of review quality are described below.
The relationship between systematic review reporting quality and study quality a
| Reporting quality . | Study quality . | |
|---|---|---|
| Good | Flawed | |
| Clear | May be helpful for clinical practice | At least you can tell it is flawed and make a judgement on utility |
| Poor | A sparkling diamond – but how do you know? | Difficult to distinguish from a good but poorly reported study |
| Reporting quality . | Study quality . | |
|---|---|---|
| Good | Flawed | |
| Clear | May be helpful for clinical practice | At least you can tell it is flawed and make a judgement on utility |
| Poor | A sparkling diamond – but how do you know? | Difficult to distinguish from a good but poorly reported study |
Adapted with permission from Williams. 21
Review the quality of the article and the depth of the review question
Distinct from quality of reporting completeness, assessing the review's quality allows for assessment of the overall clinical meaningfulness of the results. Does the PICO make sense in respect to this? The AMSTAR 2 critical appraisal instrument is useful in determining quantitative systematic review quality. 13 This checklist marks the key aspects of a systematic review and computes an outcome of the review quality. 14 If meta-analysis was performed, did the authors justify and use appropriate methods for statistical combination of results? Were weighted techniques used to combine results and adjusted for heterogeneity, if present? If heterogeneity was present, were sources of this investigated? Did authors assess the potential impact of the individual study’s risk of bias (RoB) and perform analysis to investigate the impact of RoB on the summary estimates of affect? See Table 2 for an example of a completed AMSTAR 2 checklist on a recently published poor-quality systematic review. 15
An example of assessment of the quality of a systematic review (Drake et al. ) 15 using the AMSTAR 2 checklist an explanation of which can be found at https://amstar.ca/Amstar-2.php
| Checklist item . | Response . |
|---|---|
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | No |
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | No |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | No |
| 4. Did the review authors use a comprehensive literature search strategy? | Partial Yes |
| 5. Did the review authors perform study selection in duplicate? | Yes |
| 6. Did the review authors perform data extraction in duplicate? | Yes |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? | No |
| 8. Did the review authors describe the included studies in adequate detail? | No |
| 9. Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? | No |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | No |
| 11. If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | N/A |
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | N/A |
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review | No |
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | No |
| 15. If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | N/A |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | Yes |
| Checklist item . | Response . |
|---|---|
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | No |
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | No |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | No |
| 4. Did the review authors use a comprehensive literature search strategy? | Partial Yes |
| 5. Did the review authors perform study selection in duplicate? | Yes |
| 6. Did the review authors perform data extraction in duplicate? | Yes |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? | No |
| 8. Did the review authors describe the included studies in adequate detail? | No |
| 9. Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? | No |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | No |
| 11. If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | N/A |
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | N/A |
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review | No |
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | No |
| 15. If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | N/A |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | Yes |
N/A, not applicable; PICO, population, intervention, comparator and outcome. a Denotes AMSTAR 2 critical domain. The overall confidence in the results of the review is dependent on such critical domains. When one critical domain is not satisfied, the confidence is rated as ‘low’ and the review may not provide an accurate and comprehensive summary of the available studies that address the question of interest. When more than one critical domain are not satisfied, the confidence in the results of the review is rated as ‘critically low’ and the review should not be relied on to provide an accurate and comprehensive summary of the available studies.
Quality checklists for assessment of qualitative research include Consolidated Criteria for Reporting Qualitative research (COREQ), Standards for Reporting Qualitative Research (SRQR) and Critical Appraisal Skills Programme (CASP). 16 Such checklists aim to improve identification of high-quality qualitative research in journal articles, as well as acting as a guide for conducting research. 16
Consider the authors’ interpretation and assess for spin
Spin is a distorted interpretation of results. This manifests itself in studies as (i) misleading reporting, (ii) misleading interpretation, and (iii) inappropriate extrapolation. 14 Are the conclusion’s clinical practice recommendations not supported by the studies’ findings? Is the title misleading? Is there selective reporting? These are the three most severe forms of spin occurring in systematic reviews. 17
Summarize and come to a position
Summarize the reviews main positives and negatives and establish if there is sufficient quality to merit changing clinical practice, or are fatal flaws present that nullify the review’s clinical utility? Consider internal validity (are the results true?) and external validity (are the results applicable to my patient group?). When applying the systematic review results to a particular patient, it may help to consider these points: (i) how similar are the study participants to my patient?; (ii) do the outcomes make sense to me?; (iii) what was the magnitude of treatment benefit? – work out the number needed to treat; 18 (iv) what are the adverse events?; and (v) what are my patient's values and preferences? 19
Although systematic reviews have potential for summarizing evidence for dermatological interventions in a systematic and unbiased way, the rapid expansion of poorly reported and poor-quality reviews (Table 3 ) is regrettable. We do not claim our checklist items (Table 4 ) are superior to other checklists such as those suggested by CASP, 20 but they are based on the practical experience of critical appraisal of dermatology systematic reviews conducted by the authors.
The top seven ‘sins’ of dermatology systematic reviews a
| Systematic review sin type . | Explanation . | Solution . |
|---|---|---|
| Superficial | Narrow minor topics of questionable clinical value that are often done as student projects | Senior authors to show more responsibility and undertake some form of prioritization exercises with patients and clinicians |
| Salami | Chopping up a topic into several smaller pieces in order to obtain as many publications as possible | Editors to spot and decline potential salami topics and encourage broader reviews |
| Selective | Failing to register the protocol for a systematic review and only reporting the outcomes that look interesting | Funders and journals to make prospective registration on PROSPERO mandatory |
| Sloppy | Poorly reported reviews that fail to comply with basic PRISMA reporting guidance | Journal editors to require authors to complete PRISMA checklist and to check those responses |
| Seen before | Covert duplication of existing reviews | Readers to expose and journals to investigate and retract if response inadequate |
| Specious | Reviews that give an air or spurious precision by presenting lots of numbers and statistical methods yet fail to engage with content expertise to make any sense of the topic | Review teams to include content experts Journals to employ an associate editor with systematic review expertise |
| Seriously wrong | Sausage factory reviews that get past journal editors, but which contain serious errors such as including the same study more than once in a meta-analysis | All systematic reviews with meta-analysis should be sent for statistical and content expertise review |
| Systematic review sin type . | Explanation . | Solution . |
|---|---|---|
| Superficial | Narrow minor topics of questionable clinical value that are often done as student projects | Senior authors to show more responsibility and undertake some form of prioritization exercises with patients and clinicians |
| Salami | Chopping up a topic into several smaller pieces in order to obtain as many publications as possible | Editors to spot and decline potential salami topics and encourage broader reviews |
| Selective | Failing to register the protocol for a systematic review and only reporting the outcomes that look interesting | Funders and journals to make prospective registration on PROSPERO mandatory |
| Sloppy | Poorly reported reviews that fail to comply with basic PRISMA reporting guidance | Journal editors to require authors to complete PRISMA checklist and to check those responses |
| Seen before | Covert duplication of existing reviews | Readers to expose and journals to investigate and retract if response inadequate |
| Specious | Reviews that give an air or spurious precision by presenting lots of numbers and statistical methods yet fail to engage with content expertise to make any sense of the topic | Review teams to include content experts Journals to employ an associate editor with systematic review expertise |
| Seriously wrong | Sausage factory reviews that get past journal editors, but which contain serious errors such as including the same study more than once in a meta-analysis | All systematic reviews with meta-analysis should be sent for statistical and content expertise review |
Adapted with permission from Williams. 10
Checklist of questions, considerations and tips for critical appraisal of systematic reviews
| Item . | Comments . |
|---|---|
| Is there a clear PICO and is it relevant to clinical practice? Is it clear and appropriate? | |
| Are there any conflicts of interests or financial considerations? Does the introduction provide a compelling reason for the systematic review to be performed? Are there other similar systematic reviews, perhaps not even referenced in this paper? | |
| Is this systematic review registered on PROSPERO? Was the protocol adhered to and if not, was this justified? | |
| Has a PRISMA checklist been completed and is this accurate? Pay particular attention to reporting of bias | |
| Consider using a formal checklist, e.g. AMSTAR 2 If meta-analysis was performed, was it appropriate to combine the studies? Were weighted techniques used to combine study results and adjusted for heterogeneity if present? If heterogeneity was present were sources of this investigated? Did authors assess the potential impact of risk of bias from individual studies? | |
| Do the conclusions correlate with the results? (If not, is there misleading reporting, misleading interpretation, inappropriate extrapolation?) Do the authors make recommendations for clinical practice which are not supported by the study’s findings? Is the title misleading? Is there evidence of selective reporting? | |
| What are the main positives and negatives? Consider the internal validity; are the results true? If they are true; consider external validity; are the (true) results applicable to my patient group? How similar are the study participants to my patient? Do the outcomes make sense to me? What was the magnitude of treatment effects? (Calculate NNT) What were the adverse events? What are my patients’ values and preferences? |
| Item . | Comments . |
|---|---|
| Is there a clear PICO and is it relevant to clinical practice? Is it clear and appropriate? | |
| Are there any conflicts of interests or financial considerations? Does the introduction provide a compelling reason for the systematic review to be performed? Are there other similar systematic reviews, perhaps not even referenced in this paper? | |
| Is this systematic review registered on PROSPERO? Was the protocol adhered to and if not, was this justified? | |
| Has a PRISMA checklist been completed and is this accurate? Pay particular attention to reporting of bias | |
| Consider using a formal checklist, e.g. AMSTAR 2 If meta-analysis was performed, was it appropriate to combine the studies? Were weighted techniques used to combine study results and adjusted for heterogeneity if present? If heterogeneity was present were sources of this investigated? Did authors assess the potential impact of risk of bias from individual studies? | |
| Do the conclusions correlate with the results? (If not, is there misleading reporting, misleading interpretation, inappropriate extrapolation?) Do the authors make recommendations for clinical practice which are not supported by the study’s findings? Is the title misleading? Is there evidence of selective reporting? | |
| What are the main positives and negatives? Consider the internal validity; are the results true? If they are true; consider external validity; are the (true) results applicable to my patient group? How similar are the study participants to my patient? Do the outcomes make sense to me? What was the magnitude of treatment effects? (Calculate NNT) What were the adverse events? What are my patients’ values and preferences? |
NNT, number needed to treat.
Considering each question suggested in our checklist when faced with yet another systematic review draws a timely conclusion on its quality and application to clinical practice, when acting as a reviewer or reader. Although the checklist may sound exhaustive and time-consuming, we recommend cutting it short if there are major red flags early on, such as absence of a protocol or assessment of RoB. Given the growing number of systematic reviews, having an efficient and succinct aide for appraising articles saves the reader time and energy, while simplifying the decision regarding what merits a change in clinical practice. Our intention is not to criticize others’ well-intentioned efforts, but to improve standards of reliable evidence to inform patient care.
Systematic reviews of randomized controlled trials offer one of the best methods to summarize the evidence surrounding therapeutic interventions for skin conditions.
The number of systematic reviews in the dermatology literature is increasing rapidly.
The quality of dermatology systematic reviews is generally poor.
We describe a checklist for the busy clinician or reviewer to consider when faced with a systematic review.
Key factors to consider include: determining the review motivation, establishing if the study protocol was prepublished, assessing quality of reporting and study quality using PRISMA, and AMSTAR 2 critical appraisal checklists, and assessing for evidence of spin.
Summarizing the main qualities and limitations of a systematic review will help to determine if the review is robust enough to potentially change clinical practice for patient benefit.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
No new data generated.
Ethical approval: not applicable. Informed consent: not applicable.
Moher D , Liberati A , Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement . Ann Intern Med 2009 ; 151 : 264 – 9 .
Google Scholar
Murad MH , Asi N , Alsawas M , Alahdab F . New evidence pyramid . Evid Based Med 2016 ; 21 : 125 – 7 .
Page MJ , McKenzie JE , Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . BMJ 2021 ; 372 : n71 .
Ioannidis JP . The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses . Milbank Q 2016 ; 94 : 485 – 514 .
Croitoru DO , Huang Y , Kurdina A et al. Quality of reporting in systematic reviews published in dermatology journals . Br J Dermatol 2020 ; 182 : 1469 – 76 .
Smires S , Afach S , Mazaud C et al. Quality and reporting completeness of systematic reviews and meta-analyses in dermatology . J Invest Dermatol 2021 ; 141 : 64 – 71 .
Baraldi JH , Picozzo SA , Arnold JC et al. A cross-sectional examination of conflict-of-interest disclosures of physician-authors publishing in high-impact US medical journals . BMJ Open 2022 ; 12 : e057598 .
Centers for Medicare & Medicaid Services . Open Payments Search Tool. About. Available at : https://openpaymentsdata.cms.gov/about (last accessed 22 April 2023).
Guelimi R , Afach S , Régnaux JP et al. Overlapping network meta-analyses on psoriasis systemic treatments, an overview: quantity does not make quality . Br J Dermatol 2022 ; 187 : 29 – 41 .
Williams HC . Are dermatology systematic reviews spinning out of control? Dermatology 2021 ; 237 : 493 – 5 .
National Institute for Health and Care Research . About Prospero. Available at : https://www.crd.york.ac.uk/prospero/#aboutpage (last accessed 22 April 2023).
Barbieri JS , Wehner MR . Systematic reviews in dermatology: opportunities for improvement . Br J Dermatol 2020 ; 182 : 1329 – 30 .
Shea BJ , Reeves BC , Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both . BMJ 2017 ; 358 : j4008 .
AMSTAR . AMSTAR checklist. Available at : https://amstar.ca/Amstar_Checklist.php (last accessed 22 April 2023).
Drake L , Reyes-Hadsall S , Martinez J et al. Evaluation of the safety and effectiveness of nutritional supplements for treating hair loss: a systematic review . JAMA Dermatol 2023 ; 159 : 79 – 86 .
Stenfors T , Kajamaa A , Bennett D . How to … assess the quality of qualitative research . Clin Teach 2020 ; 17 : 596 – 9 .
Yavchitz A , Ravaud P , Altman DG et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity . J Clin Epidemiol 2016 ; 75 : 56 – 65 .
Manriquez JJ , Villouta MF , Williams HC . Evidence-based dermatology: number needed to treat and its relation to other risk measures . J Am Acad Dermatol 2007 ; 56 : 664 – 71 .
Williams HC . Applying trial evidence back to the patient . Arch Dermatol 2003 ; 139 : 1195 – 200 .
CASP . CASP checklists. Available at : https://casp-uk.net/casp-tools-checklists/ (last accessed 22 April 2023).
Williams HC . Cars, CONSORT 2010, and clinical practice . Trials 2010 ; 11 : 33 .
Learning objective
To demonstrate up-to-date knowledge on assessing systematic reviews.
Which of the following critical appraisal checklists is useful for assessment of items that should be reported in a systematic review?
Which one of the following statements is correct?
The number of published systematic reviews in the dermatology literature is falling.
The quality of published dermatology systematic reviews is generally very good.
Publishing details of the PRISMA checklist in a systematic review indicates that the study quality is high.
External validity refers to the applicability of results to your patient group.
Internal validity refers to the applicability of results to your patient group.
Spin in systematic reviews can be described by which one of the following measures?
Authors declaring all conflicts of interest.
Title suggesting beneficial effect not supported by findings.
Adequate reporting of study limitations.
Conclusion formulating recommendations for clinical practice supported by findings.
Reporting a departure from study protocol that may modify interpretation of results.
PICO stands for which of the following.
PubMed, inclusion, comparator, outcome.
Population, items, comparator, outcome.
Population, intervention, context, observations.
Protocol, intervention, certainty, outcome.
Population, intervention, comparator, outcome.
Publication of a systematic review study protocol can be found at which source?
Cochrane Library.
ClinicalTrials.gov.
This learning activity is freely available online at https://oupce.rievent.com/a/TWWDCK
Users are encouraged to
Read the article in print or online, paying particular attention to the learning points and any author conflict of interest disclosures.
Reflect on the article.
Register or login online at https://oupce.rievent.com/a/TWWDCK and answer the CPD questions.
Complete the required evaluation component of the activity.
Once the test is passed, you will receive a certificate and the learning activity can be added to your RCP CPD diary as a self-certified entry.
This activity will be available for CPD credit for 5 years following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional period.
Author notes
| Month: | Total Views: |
|---|---|
| April 2023 | 10 |
| May 2023 | 63 |
| June 2023 | 63 |
| July 2023 | 119 |
| August 2023 | 194 |
| September 2023 | 214 |
| October 2023 | 250 |
| November 2023 | 189 |
| December 2023 | 205 |
| January 2024 | 336 |
| February 2024 | 375 |
| March 2024 | 461 |
| April 2024 | 623 |
| May 2024 | 612 |
| June 2024 | 340 |
| July 2024 | 73 |
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1365-2230
- Copyright © 2024 British Association of Dermatologists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Critical Appraisal of a Systematic Review: A Concise Review
Affiliations.
- 1 Division of Pulmonary and Critical Care Medicine, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI.
- 2 Department of Anesthesiology, University Hospital RWTH Aachen University, Aachen, Germany.
- 3 Department of Intensive Care Medicine, University Hospital RWTH Aachen University, Aachen, Germany.
- 4 Department of Anesthesiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia.
- 5 Clinical Evaluation Research Unit, Department of Critical Care Medicine, Queen's University, KGH Research Institute, Kingston Health Sciences Centre, Kingston, ON, Canada.
- 6 Department of Anesthesiology and Intensive Care Medicine, University Hospital Wuerzburg, Wuerzburg, Germany.
- PMID: 35853198
- DOI: 10.1097/CCM.0000000000005602
Objectives: Concise definitive review of how to read and critically appraise a systematic review.
Data sources: None.
Study selection: Current literature describing the conduct, reporting, and appraisal of systematic reviews and meta-analyses.
Data extraction: Best practices for conducting, reporting, and appraising systematic review were summarized.
Data synthesis: A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant original research, and to collect and analyze data from the studies that are included in the review. Critical appraisal methods address both the credibility (quality of conduct) and rate the confidence in the quality of summarized evidence from a systematic review. The A Measurement Tool to Assess Systematic Reviews-2 tool is a widely used practical tool to appraise the conduct of a systematic review. Confidence in estimates of effect is determined by assessing for risk of bias, inconsistency of results, imprecision, indirectness of evidence, and publication bias.
Conclusions: Systematic reviews are transparent and reproducible summaries of research and conclusions drawn from them are only as credible and reliable as their development process and the studies which form the systematic review. Applying evidence from a systematic review to patient care considers whether the results can be directly applied, whether all important outcomes have been considered, and if the benefits are worth potential harms and costs.
Copyright © 2022 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
PubMed Disclaimer
Conflict of interest statement
Dr. Hill’s institution received funding from Fresenius Kabi and the Medical Faculty Rheinisch-Westfälische Technische Hochschule Aachen; she received funding from Fresenius Kabi. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Similar articles
- How to Critically Appraise and Interpret Systematic Reviews and Meta-Analyses of Diagnostic Accuracy: A User Guide. Frank RA, Salameh JP, Islam N, Yang B, Murad MH, Mustafa R, Leeflang M, Bossuyt PM, Takwoingi Y, Whiting P, Dawit H, Kang SK, Ebrahimzadeh S, Levis B, Hutton B, McInnes MDF. Frank RA, et al. Radiology. 2023 May;307(3):e221437. doi: 10.1148/radiol.221437. Epub 2023 Mar 14. Radiology. 2023. PMID: 36916896 Free PMC article. Review.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- Characteristics, quality and volume of the first 5 months of the COVID-19 evidence synthesis infodemic: a meta-research study. Abbott R, Bethel A, Rogers M, Whear R, Orr N, Shaw L, Stein K, Thompson Coon J. Abbott R, et al. BMJ Evid Based Med. 2022 Jun;27(3):169-177. doi: 10.1136/bmjebm-2021-111710. Epub 2021 Jun 3. BMJ Evid Based Med. 2022. PMID: 34083212 Free PMC article. Review.
- Behavioural interventions for smoking cessation: an overview and network meta-analysis. Hartmann-Boyce J, Livingstone-Banks J, Ordóñez-Mena JM, Fanshawe TR, Lindson N, Freeman SC, Sutton AJ, Theodoulou A, Aveyard P. Hartmann-Boyce J, et al. Cochrane Database Syst Rev. 2021 Jan 4;1:CD013229. doi: 10.1002/14651858.CD013229.pub2. Cochrane Database Syst Rev. 2021. PMID: 33411338
- The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
- Advantages of unilateral percutaneous kyphoplasty for osteoporotic vertebral compression fractures-a systematic review and meta-analysis. Cao DH, Gu WB, Zhao HY, Hu JL, Yuan HF. Cao DH, et al. Arch Osteoporos. 2024 May 15;19(1):38. doi: 10.1007/s11657-024-01400-8. Arch Osteoporos. 2024. PMID: 38750277 Review.
- Evaluating COVID-19 vaccine acceptance among parents in Saudi Arabia: a systematic review examining attitudes, hesitancy, and intentions. Sayed AA. Sayed AA. Front Public Health. 2024 Mar 22;12:1327944. doi: 10.3389/fpubh.2024.1327944. eCollection 2024. Front Public Health. 2024. PMID: 38584927 Free PMC article.
- Utilization of Mirror Visual Feedback for Upper Limb Function in Poststroke Patients: A Systematic Review and Meta-Analysis. Kim H, Lee E, Jung J, Lee S. Kim H, et al. Vision (Basel). 2023 Nov 15;7(4):75. doi: 10.3390/vision7040075. Vision (Basel). 2023. PMID: 37987295 Free PMC article. Review.
- Comparison of clinical outcomes between aggressive and non-aggressive intravenous hydration for acute pancreatitis: a systematic review and meta-analysis. Li XW, Wang CH, Dai JW, Tsao SH, Wang PH, Tai CC, Chien RN, Shao SC, Lai EC. Li XW, et al. Crit Care. 2023 Mar 22;27(1):122. doi: 10.1186/s13054-023-04401-0. Crit Care. 2023. PMID: 36949459 Free PMC article.
- Evidence-Based Medicine Working Group: Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992; 268:2420–2425
- Guyatt G: Users’ Guides to the Medical Literature. Third Edition. New York, NY, McGraw Hill Education, 2015
- Djulbegovic B, Guyatt GH: Progress in evidence-based medicine: A quarter century on. Lancet 2017; 390:415–423
- Doig GS, Roberts I, Bellomo R: The tens of thousands of lives saved by randomized clinical trials in critical care. Intensive Care Med 2015; 41:701–704
- Davidoff F, Haynes B, Sackett D, et al.: Evidence based medicine. BMJ 1995; 310:1085–1086
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ingenta plc
- Ovid Technologies, Inc.
- Wolters Kluwer

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Shopping Cart
No products in the cart.

Critical Appraisal? An Overview of Why and How
Date of live webinar: august 7, 2024, 1:00pm-2:30pm, central time..
The need for critical appraisal skills and the ability to teach them is expanding rapidly. Gain foundational critical appraisal skills that set you on the path of increasing your value to your institution and improving your career prospects.
Critical appraisal is the process of systematically evaluating the methodology and conclusions of research studies. Librarians skilled in the area are able to critically read and evaluate health care literature and collaborate with students, health care providers, and researchers to support medical research and the practice of medicine. The need for critical appraisal skills and the ability to teach them is expanding rapidly, affording you opportunities to increase your value to your institution and improve your career prospects.
Learning Outcomes
By the end of this course, you will be able to:
- Define and contextualize critical appraisal within the EBM cycle
- Name the four explanations for any study finding
- Identify different critical appraisal needs at their institution
- Describe librarian roles in delivering critically appraised information
- List two sources of critical appraisal tools.
- Demonstrate beginner level ability to critically appraise an article.
Audience
Medical librarians and other information professionals who work with health professionals, students, residents, or researchers.

Marie Ascher, MS, MPH is the Director of the Phillip Capozzi, MD Library at New York Medical College, a medical librarian for over 30 years, with a particular interest in public health and epidemiology. She is the course director for the Critical Appraisal Institute for Librarians CE course and on the faculty of the department of Epidemiology, and the School of Medicine and teaches Evidence Based Practice at New York Medical College.

Abraham Wheeler, AHIP , is a health sciences librarian at Michigan State University, in which capacity he works with the MSU Institute for Health Policy and is the EBM content expert for the College of Osteopathic Medicine and the PA Program. He also works with the Flint Research Group, the Department of Biostatistics & Epidemiology, and the MSU Institute for Global Health Studies. His work focuses on evidence-based health services policy recommendations. He is a Critical Appraisal Institute for Librarians (CAIFL) instructor and has taught several well-reviewed MLA courses and webinars on EBP and critical appraisal.
Registration Information
- Length: 1.5 hour webinar
- Date: August 7, 2024, 1:00pm-2:30pm, Central Time
- Technical information: The login will be sent in advance to access the live webinar, resources, evaluation, and certificate.
- Register, participate, and earn 1.5 MLA continuing education (CE) contact hours.
Course Content


Course Includes
- Course Certificate
Email Address
Remember Me
Registration confirmation will be emailed to you.
- Open access
- Published: 02 July 2024
Unravelling the complexity of ventilator-associated pneumonia: a systematic methodological literature review of diagnostic criteria and definitions used in clinical research
- Markus Fally 1 ,
- Faiuna Haseeb 2 , 3 ,
- Ahmed Kouta 2 , 3 ,
- Jan Hansel 3 , 4 ,
- Rebecca C. Robey 2 , 3 ,
- Thomas Williams 5 ,
- Tobias Welte 6 ,
- Timothy Felton 2 , 3 , 5 &
- Alexander G. Mathioudakis 2 , 3
Critical Care volume 28 , Article number: 214 ( 2024 ) Cite this article
1978 Accesses
58 Altmetric
Metrics details
Ventilator-associated pneumonia (VAP) is a prevalent and grave hospital-acquired infection that affects mechanically ventilated patients. Diverse diagnostic criteria can significantly affect VAP research by complicating the identification and management of the condition, which may also impact clinical management.
We conducted this review to assess the diagnostic criteria and the definitions of the term “ventilator-associated” used in randomised controlled trials (RCTs) of VAP management.
Search methods
Based on the protocol (PROSPERO 2019 CRD42019147411), we conducted a systematic search on MEDLINE/PubMed and Cochrane CENTRAL for RCTs, published or registered between 2010 and 2024.
Selection criteria
We included completed and ongoing RCTs that assessed pharmacological or non-pharmacological interventions in adults with VAP.
Data collection and synthesis
Data were collected using a tested extraction sheet, as endorsed by the Cochrane Collaboration. After cross-checking, data were summarised in a narrative and tabular form.
In total, 7,173 records were identified through the literature search. Following the exclusion of records that did not meet the eligibility criteria, 119 studies were included. Diagnostic criteria were provided in 51.2% of studies, and the term “ventilator-associated” was defined in 52.1% of studies. The most frequently included diagnostic criteria were pulmonary infiltrates (96.7%), fever (86.9%), hypothermia (49.1%), sputum (70.5%), and hypoxia (32.8%). The different criteria were used in 38 combinations across studies. The term “ventilator-associated” was defined in nine different ways.
Conclusions
When provided, diagnostic criteria and definitions of VAP in RCTs display notable variability. Continuous efforts to harmonise VAP diagnostic criteria in future clinical trials are crucial to improve quality of care, enable accurate epidemiological assessments, and guide effective antimicrobial stewardship.
Ventilator-associated pneumonia (VAP) stands as the most prevalent and serious hospital-acquired infection observed in intensive care units [ 1 ]. VAP prolongs hospital stays, durations of mechanical ventilation, and is associated with considerable mortality and an increase in healthcare costs [ 2 , 3 ].
Diagnosing VAP can be challenging for clinicians as it shares clinical signs and symptoms with other forms of pneumonia as well as non-infectious conditions [ 4 ]. The most recent international clinical guidelines define VAP as the presence of respiratory infection signs combined with new radiographic infiltrates in a patient who has been ventilated for at least 48 h [ 5 , 6 ]. While the guidelines developed by ERS/ESICM/ESCMID/ALAT do not provide a detailed definition of signs of respiratory infection [ 5 ], the ATS/IDSA guidelines mention that clinical signs may include the new onset of fever, purulent sputum, leucocytosis, and decline in oxygenation [ 6 ]. However, the ATS/IDSA guideline panel also acknowledges that there is no gold standard for the diagnosis of VAP [ 6 ]. This lack of a standardised definition is further highlighted by the varying, surveillance-based definitions of VAP provided by the Centre for Disease Control (CDC) and the European Centre for Disease Control (ECDC) [ 7 , 8 ]. These definitions, focusing on a combination of clinical, radiological, and microbiological signs to identify cases of VAP, were established to standardise reporting and facilitate the monitoring of infections in healthcare settings. However, the criteria given by the CDC and ECDC may not always align with the diagnostic criteria used by clinicians to confirm or rule out the condition [ 9 , 10 , 11 ].
Variations in the eligibility criteria applied to VAP can have a significant impact on systematic reviews and meta-analyses that assess different interventions, primarily due to the potential lack of comparability among the studied populations [ 12 ]. Furthermore, the incidence of VAP may be underestimated when excessively strict diagnostic criteria are employed [ 13 , 14 ].
A recent systematic review conducted by Weiss et al. focused on inclusion and judgment criteria used in randomised controlled trials (RCTs) on nosocomial pneumonia and found considerable heterogeneity [ 15 ]. However, the authors only considered RCTs evaluating antimicrobial treatment as interventions, did not distinguish between hospital-acquired pneumonia (HAP) and VAP, and did not evaluate definitions of the term "ventilator-associated".
The objective of this systematic review was to provide a concise overview of the diagnostic criteria for VAP recently used in RCTs, as well as the definitions attributed to the term "ventilator-associated". Its findings will provide valuable insights to a forthcoming task force, which aims to establish a uniform definition and diagnostic criteria for VAP in clinical trials. The task force will be made up of representatives from prominent international societies with an interest in VAP, as well as patient partners with lived experience. The harmonisation of the diagnostic criteria for VAP in upcoming clinical research are vital for enhancing patient care, enabling accurate epidemiological studies, and guiding successful antimicrobial stewardship programs.
Protocol and registration
The protocol for this systematic review was registered in advance with the International Prospective Register of Systematic Reviews (PROSPERO 2019 CRD42019147411), encompassing a broad review focusing on pneumonia outcomes and diagnostic criteria in RCTs. Recognising the limitations of discussing all findings in one manuscript, we opted to produce several focused and comprehensive manuscripts, all employing the same fundamental methodology, as registered with PROSPERO. While a previous publication focused on outcomes reported in RCTs on pneumonia management [ 16 ], the current submission specifically addresses diagnostic criteria for VAP.
Eligibility criteria
We included RCTs that were registered, planned, and/or completed that: (1) enrolled adults with VAP; and (2) assessed the safety, efficacy and/or effectiveness of pharmacological or non-pharmacological interventions for treating VAP.
We have excluded systematic reviews, meta-analyses, narrative reviews, post hoc analyses from RCTs, observational studies, case reports, editorials, conference proceedings, and studies that do not exclusively focus on pneumonia (such as trials including patients with pneumonia alongside other diseases). Additionally, studies on pneumonia subtypes other than VAP, such as pneumonia without specifying a subtype, community-acquired pneumonia (CAP), healthcare-associated pneumonia (HCAP), and HAP, have also been excluded. To maintain focus and relevance, studies on Coronavirus Disease 2019 (COVID-19) were excluded from this systematic review, as the viral aetiology and distinct clinical management protocols differ significantly from the nature and treatment strategies of VAP. RCT protocols were only included if the results have not been previously published in another article included in this systematic review. Due to resource constraints and the lack of multilingual expertise within the review team, this systematic review was restricted to English-language RCTs.
Information sources and search
On 20 May 2024, we searched MEDLINE/PubMed, and the Cochrane Register of Controlled Trials (CENTRAL) for RCTs published between 1 January 2010 and 19 May 2024. We used electronic algorithms introducing a combination of controlled vocabulary and search terms as reported in the Appendix.
Study selection
Two reviewers (FH, MF) independently screened titles and abstracts to identify eligible studies using Rayyan [ 17 ]. In case of disagreement, a third reviewer was consulted (AGM). After immediate exclusion of duplicates using EndNote X9, four reviewers (AGM, FH, JH, MF) independently checked for eligibility at full-text level. The results of the selection process are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 18 ].
Data collection process
We developed an extraction sheet as endorsed by the Cochrane Collaboration [ 19 ]. The extraction sheet was independently tested by three reviewers (AGM, FH, MF) on five randomly selected studies and adapted to ensure good inter-reviewer agreement. The extraction sheet contained the following elements: (1) study ID, name, reference and NCT number; (2) type of pneumonia: CAP, HCAP, HAP and/or VAP; (3) diagnostic criteria for pneumonia; (4) definition of setting; (5) study origin, design, populations, interventions, and outcomes.
Four reviewers (AGM, FH, JH, MF) extracted data from the eligible studies. Data were extracted sequentially from either a manuscript containing published results, a published protocol, or, upon obtaining a trial registration number from CENTRAL, from one of the designated trial registries, such as ClinicalTrials.gov, the Clinical Trials Registry India (CTRI), the Chinese Clinical Trial Registry (ChiCTR), the European Clinical Trials Database (EudraCT), the Iranian Registry of Clinical Trials (IRCT), the Japan Primary Registries Network (JPRN), and the Japanese University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR). Cross-checking of all extracted data was performed by a second reviewer (AGM, AK, MF, RR, TW). Disagreements regarding data collection were resolved by discussion between all reviewers.
Synthesis of results
The findings were consolidated through a combination of narrative and tabular formats. The presentation encompassed the quantitative representation of each diagnostic criterion in terms of numerical values and proportions. Additionally, we provide an analysis of the various combinations of diagnostic criteria employed in RCTs in a sunburst diagram and a tabular format, along with an examination of the definitions attributed to the term "ventilator-associated".
Risk of bias
The main goal of this systematic review was to explore the diagnostic criteria used in clinical trials for diagnosing VAP. It covered trials with published protocols and/or results, as well as those only registered in a trial database. The varying levels and gaps in the information provided by the various sources made it difficult to conduct a reliable and meaningful risk of bias assessment for all included studies. However, for RCTs with published data, risk of bias was evaluated by four reviewers (AGM, JH, MF, RR) using the Risk of Bias in Randomized Trials 2 tool (RoB-2 tool), as endorsed by the Cochrane Collaboration [ 20 ].
Study selection and characteristics
A total of 7173 records were identified through the databases MEDLINE and CENTRAL, as illustrated in Fig. 1 . Following the removal of duplicate entries, a screening process involving the evaluation of titles and abstracts was conducted on 5652 records. Among these, 650 records were deemed potentially eligible for inclusion. Ultimately, our review included 119 studies that specifically focused on VAP (Table S1 in the Appendix, the full dataset is available online [ 21 ]).

PRISMA flowchart showing study selection
The total number of patients in the 119 identified studies was 21,289. Among these studies, 83 focused exclusively on VAP, while the remaining studies encompassed various subtypes of pneumonia in addition to VAP (see Table 1 ). The majority of these studies were registered, and their protocols were accessible either through publication in a journal article or on a clinical trial platform. Results were accessible in 56.3% of cases, while both results and the protocol were accessible in 36.9% of cases. In 40.3% of the included studies, data could only be obtained from a trial registry platform, with ClinicalTrials.gov being the primary platform in 36 out of 48 cases, and ChiCTR (n = 2), CTRI (n = 3), EudraCT (n = 3), IRCT (n = 2), JPRN (n = 1) and UMIN-CTR (n = 1) in the remaining cases.
Diagnostic criteria were provided in 51.2% and the term “ventilator-associated” was defined in 52.1% of the studies, respectively. Of the 20 studies (16.8%) that referred to previously published diagnostic criteria, 13 cited the Clinical Pulmonary Infection Score (CPIS) [ 22 ], while the remaining referred to national and international guidelines.
We evaluated the risk of bias in 67 studies with published results using the RoB-2 tool. The overall assessment showed that 25% of the studies were at high risk of bias, 30% were at low risk of bias, and the remaining 45% had some concerns about potential bias. These results indicate variability in the methodological quality of the studies included in the review. The overall risk of bias and the detailed results of our assessments for the 67 studies are displayed in the Appendix (Figures SF1-SF2).
Diagnostic criteria for VAP
Pulmonary infiltrates.
Of the 61 studies on VAP that provided diagnostic criteria, 59 (96.7%) included the radiological evidence of a new or progressive pulmonary infiltrate.
Clinical signs and symptoms
The most frequently included clinical signs and symptoms were fever (86.9%), hypothermia (49.1%), sputum (70.5%), and hypoxia (32.8%). Different cut-off values were employed to define fever and hypothermia, as indicated in Table 2 . The majority of studies, accounting for 45.2%, utilised a cut-off of > 38 degrees Celsius (°C) to define fever, while 13.2% of studies used a cut-off of ≥ 38°C. In the case of hypothermia, the most commonly employed cut-off value was < 35°C, which was utilised in 43.3% of studies that included hypothermia as a criterion. Only a minority of studies provided information on the site of temperature measurement. Oral measurement was the most frequently employed method, followed by axillary and core temperature measurements (further details are displayed in Table S2 in the Appendix).
Biochemistry criteria
Fifty-four studies (88.5%) incorporated white blood count abnormalities as part of their diagnostic criteria for VAP. Conversely, only one study included an elevation of procalcitonin (PCT) as a diagnostic factor, and none of the identified studies included C-reactive protein (CRP). The specific thresholds for leucocytosis and leucopoenia varied across studies, with leucocyte counts ranging from greater than 10,000/mm3 to greater than 12,000/mm3 for leucocytosis, and less than 3,500/mm3 to less than 4,500/mm3 for leucopoenia (Table 3 ).
Combinations of diagnostic criteria
All definitions of pneumonia were composite in nature and required the fulfilment of a minimum number of predetermined criteria for the diagnosis to be established. In 90.2% of the studies the presence of a new pulmonary infiltrate was a mandatory criterion. Two studies did not include an infiltrate as criterion, whereas the remaining studies (n = 4) included the presence of an infiltrate in their criteria, it was, however, not required for a diagnosis.
The most commonly employed set of diagnostic criteria (18/61, 29.5%) consisted of a pulmonary infiltrate along with two or more additional criteria. However, these additional criteria varied across studies (Fig. 2 ). A quarter (17/61) of the included studies that provided diagnostic criteria required the fulfilment of all individual criteria for diagnosis, including an infiltrate. An infiltrate and one or more additional criteria were used to establish a diagnosis of VAP in 14.8% of studies (9/61). A total of 38 different combinations of diagnostic criteria for VAP were used in the 61 identified studies. A full set of these criteria is displayed in Table S3 in the Appendix.

The different combinations of diagnostic criteria used in VAP RCTs. CXR radiological evidence of a new infiltrate; T temperature criterion; WBC white blood count criterion; dys/tach dyspnoea and/or tachypnoea; O2 hypoxia; auscultation auscultation abnormalities
Definition of “ventilator-associated”
We noted that 52.1% of included studies incorporated a specific definition of the term “ventilator-associated” (Table 4 ). A total of nine distinct definitions were identified across 62 RCTs. The definition most commonly used was “onset after > 48 h of mechanical ventilation” (82.3%). Other definitions employed varying time thresholds, ranging from 24 h to seven days. Additionally, certain studies introduced supplementary criteria to further delineate the concept of “ventilator-associated”, such as administration of antibiotics prior to mechanical ventilation, duration of hospitalisation, or the timing of extubation.
Summary of evidence
This systematic review provides a concise overview of the diagnostic criteria for VAP used in RCTs and the definitions attributed to the term “ventilator-associated”. A total of 119 studies on VAP, published or registered between 2010 and 2024, were included, spanning a total of 21,289 patients. The majority of studies focused exclusively on VAP, while some also included other subtypes of pneumonia alongside VAP. Diagnostic criteria were provided in only 51.2% of the studies, and the term “ventilator-associated” was defined in only 52.1% of the studies. The most commonly utilised definition for “ventilator-associated” was “onset after > 48 h of mechanical ventilation”, used by 82.3% of studies providing a definition.
In clinical practice, the diagnosis of VAP is often based on a combination of clinical signs, laboratory results, and imaging findings, yet these are not without their limitations [ 8 ]. Our systematic review revealed considerable heterogeneity among diagnostic criteria for VAP in recent RCTs. Various combinations of specific criteria were employed to define VAP, leading to significant variability. Moreover, commonly used criteria were defined in different ways, with variations observed in the thresholds set for fever/hypothermia, as well as leucocytosis/leucopoenia.
Several criteria that were used in the studies included in our review have been shown to be insufficient for confirming a diagnosis of VAP. One of the most important criteria, included in the majority of reviewed RCTs, a new or progressive pulmonary infiltrate, has previously been reported to be of limited diagnostic value due to a lack of specificity [ 14 ]. Additionally, criteria like fever/hypothermia and the measurement of biomarkers such as leukocytes, CRP, and PCT may not be effective in diagnosing or excluding VAP in various clinical settings [ 4 , 23 , 24 ]. Despite this, CRP is widely used and has demonstrated some clinical value in predicting VAP [ 25 ]. It is, therefore, surprising that none of the RCTs included in our review employed CRP as a diagnostic criterion.
Overall, the findings of our systematic review underline the diverse nature of VAP, with different diagnostic criteria increasing the risk of both over- and underdiagnosis of VAP [ 14 , 26 ]. There have been attempts to diagnose VAP more objectively, one of these being the development of the CPIS in 1991, a six-component score that 10.9% of studies included in our review referred to [ 27 ]. This score includes different cut-offs for body temperature, leucocyte counts, tracheal secretion appearances, oxygenation levels and radiographical changes to estimate the risk for VAP. However, the CPIS has been shown not to be superior to other diagnostic criteria, and, therefore, its application remains controversial [ 8 , 11 , 22 , 28 ]. Other commonly applied criteria, such as the surveillance-based criteria by the ECDC and CDC, did not seem to be accurate enough to detect true cases of VAP either [ 9 , 10 , 11 ]. Furthermore, there is limited agreement between the two surveillance-based criteria, which has previously resulted in different estimates of VAP events [ 29 ].
In lieu of definitive diagnostic scores or sets of diagnostic criteria to detect all true cases of VAP, the findings of our systematic review indicate the need for more homogeneous diagnostic criteria in future RCTs, to assure their comparability. Currently, international guidelines avoid providing clear diagnostic criteria for VAP [ 5 , 6 ]. Given the significance of establishing strong consensus definitions for high-risk conditions like VAP, it is essential to emphasise even further that a uniform definition is crucial not only for advancing therapeutic research but also, and perhaps more importantly, for refining diagnostic methods. Together with core outcome sets, these definitions can help to improve the likelihood of attaining robust and reliable findings in forthcoming systematic reviews and meta-analyses [ 16 , 30 ].
Strengths and limitations
We used a comprehensive search strategy which included multiple databases and a wide range of search terms, ensuring broad identification of all potentially relevant trials. Additionally, the inclusion criteria were clearly defined, and the study selection process was conducted independently by multiple reviewers to minimise bias. The extraction sheet used for data collection was tested for inter-reviewer agreement and adapted accordingly. Another strength is the open availability of the complete dataset, maximising the transparency and reproducibility of our findings.
However, the following limitations need to be acknowledged. Firstly, the review only included RCTs conducted in English, which may have introduced language bias. This approach was adopted to ensure feasible and reliable data analysis within the scope of the resources available.
Additionally, the exclusion of studies focusing on pneumonia subtypes other than VAP may limit the generalisability of our findings. Furthermore, the lack of diagnostic criteria and definitions in a significant proportion of included studies suggests a potential reporting bias. This might be reinforced by the fact that 40.3% of data were received from trial registry platforms. Compared to final manuscript publications, reporting of eligibility criteria is often incomplete on registry platforms, therefore this must be highlighted as a limitation [ 31 ].
This systematic review provides an overview of diagnostic criteria for VAP used in RCTs and the definitions attributed to the term “ventilator-associated”. Our findings highlight the heterogeneity and lack of standardisation in commonly used diagnostic criteria, as well as the variability in definitions of "ventilator-associated" across clinical trials. We emphasise the need for a uniform definition of VAP to enable better comparability between studies and interventions. The results of this review will inform the work of an upcoming task force aimed at establishing such standardised criteria.
Availability of data and materials
Raw data are accessible via the Open Science Framework (OSF) at osf.io/v3 × 42. This link is referenced in our manuscript (Ref. 21).
Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):28. https://doi.org/10.1038/s41572-021-00259-0 .
Article Google Scholar
Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51:S120–5. https://doi.org/10.1086/653060 .
Article PubMed Google Scholar
Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–71. https://doi.org/10.1016/S1473-3099(13)70081-1 .
Alagna L, Palomba E, Chatenoud L, et al. Comparison of multiple definitions for ventilator-associated pneumonia in patients requiring mechanical ventilation for non-pulmonary conditions: preliminary data from PULMIVAP, an Italian multi-centre cohort study. J Hosp Infect. 2023;140:90–5. https://doi.org/10.1016/j.jhin.2023.07.023 .
Article CAS PubMed Google Scholar
Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50:1700582. https://doi.org/10.1183/13993003.00582-2017 .
Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111. https://doi.org/10.1093/cid/ciw353 .
Article PubMed PubMed Central Google Scholar
Plachouras D, Lepape A, Suetens C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018;44:2216–8. https://doi.org/10.1007/s00134-018-5113-0 .
Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41:34–48. https://doi.org/10.1007/s00134-014-3564-5 .
Ramírez-Estrada S, Lagunes L, Peña-López Y, et al. Assessing predictive accuracy for outcomes of ventilator-associated events in an international cohort: the EUVAE study. Intensive Care Med. 2018;44:1212–20. https://doi.org/10.1007/s00134-018-5269-7 .
Waltrick R, Possamai DS, de Aguiar FP, et al. Comparison between a clinical diagnosis method and the surveillance technique of the Center for Disease Control and Prevention for identification of mechanical ventilator-associated pneumonia. Rev Bras Ter Intensiva. 2015;27:260. https://doi.org/10.5935/0103-507X.20150047 .
Rahimibashar F, Miller AC, Yaghoobi MH, Vahedian-Azimi A. A comparison of diagnostic algorithms and clinical parameters to diagnose ventilator-associated pneumonia: a prospective observational study. BMC Pulm Med. 2021;21:161. https://doi.org/10.1186/s12890-021-01527-1 .
Article CAS PubMed PubMed Central Google Scholar
Malmivaara A. Methodological considerations of the GRADE method. Ann Med. 2015;47:1–5.
Al-Omari B, McMeekin P, Allen AJ, et al. Systematic review of studies investigating ventilator associated pneumonia diagnostics in intensive care. BMC Pulm Med. 2021;21:196. https://doi.org/10.1186/s12890-021-01560-0 .
Fernando SM, Tran A, Cheng W, et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients—a systematic review and meta-analysis. Intensive Care Med. 2020;46:1170–9. https://doi.org/10.1007/s00134-020-06036-z .
Weiss E, Essaied W, Adrie C, et al. Treatment of severe hospital-acquired and ventilator-associated pneumonia: a systematic review of inclusion and judgment criteria used in randomized controlled trials. Crit Care. 2017. https://doi.org/10.1186/s13054-017-1755-5 .
Mathioudakis AG, Fally M, Hansel J, et al. Clinical trials of pneumonia management assess heterogeneous outcomes and measurement instruments. J Clin Epidemiol. 2023;164:88–95. https://doi.org/10.1016/j.jclinepi.2023.10.011 .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4 .
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9.
Li T, Higgins J, Deeks J (editors) (2019) Chapter 5: Collecting data | Cochrane Training. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0. https://training.cochrane.org/handbook/current/chapter-05 . Accessed 21 Jul 2020
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. https://doi.org/10.1136/bmj.l4898 .
ERS COS Pneumonia dataset. http://osf.io/v3x42
Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51:S131–5. https://doi.org/10.1086/653062 .
Huang H-B, Peng J-M, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114. https://doi.org/10.1186/s13613-017-0338-6 .
Palazzo SJ, Simpson T, Schnapp L. Biomarkers for ventilator-associated pneumonia: review of the literature. Heart Lung. 2011;40:293–8. https://doi.org/10.1016/j.hrtlng.2010.11.003 .
Póvoa P, Martin-Loeches I, Ramirez P, et al. Biomarker kinetics in the prediction of VAP diagnosis: results from the BioVAP study. Ann Intensive Care. 2016;6:32. https://doi.org/10.1186/s13613-016-0134-8 .
Johnstone J, Muscedere J, Dionne J, et al. Definitions, rates and associated mortality of ICU-acquired pneumonia: a multicenter cohort study. J Crit Care. 2023;75:154284. https://doi.org/10.1016/j.jcrc.2023.154284 .
Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–9. https://doi.org/10.1164/ajrccm/143.5_Pt_1.1121 .
Fàbregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54:867–73.
Craven TH, Wojcik G, McCoubrey J, et al. Lack of concordance between ECDC and CDC systems for surveillance of ventilator associated pneumonia. Intensive Care Med. 2018;44:265–6. https://doi.org/10.1007/s00134-017-4993-8 .
Mathioudakis AG, Khaleva E, Fally M, et al. Core outcome sets, developed collaboratively with patients, can improve the relevance and comparability of clinical trials. Eur Respir J. 2023;61:2202107. https://doi.org/10.1183/13993003.02107-2022 .
Speich B, Gloy VL, Klatte K, et al. Reliability of trial information across registries for trials with multiple registrations. JAMA Netw Open. 2021;4:e2128898. https://doi.org/10.1001/jamanetworkopen.2021.28898 .
Download references
Acknowledgements
We would like to acknowledge and honour the contributions of Prof. Tobias Welte, who was a vital member of our research team and co-author of this manuscript. Prof. Welte passed away after the initial submission of this work but before its final acceptance. His insights and expertise were invaluable to the development of this research, and he remains deeply missed by the team. We dedicate this work to his memory.
Open access funding provided by Copenhagen University This study was partly supported by the NIHR Manchester Biomedical Research Centre (BRC, NIHR203308) as well as the Capital Region of Denmark (Region Hovedstaden). The funders had no role in study design, data collection or analysis, decision to publish, nor preparation of the manuscript. Dr Jan Hansel was supported by an NIHR Academic Clinical Fellowship in Intensive Care Medicine. Dr Rebecca Robey was supported by an NIHR Academic Clinical Fellowship in Respiratory Medicine. Dr Alexander G. Mathioudakis was supported by an NIHR Clinical Lectureship in Respiratory Medicine. All authors have completed a ICMJE uniform disclosure form detailing any conflicts of interest outside the submitted work that they may have. None of the authors have conflicts directly related to this work.
Author information
Authors and affiliations.
Department of Respiratory Medicine and Infectious Diseases, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Copenhagen, Denmark
Markus Fally
North West Lung Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK
Faiuna Haseeb, Ahmed Kouta, Rebecca C. Robey, Timothy Felton & Alexander G. Mathioudakis
Division of Immunology, Immunity to Infection and Respiratory Medicine, School of Biological Sciences, The University of Manchester, Manchester, UK
Faiuna Haseeb, Ahmed Kouta, Jan Hansel, Rebecca C. Robey, Timothy Felton & Alexander G. Mathioudakis
North West School of Intensive Care Medicine, Health Education England North West, Manchester, UK
Acute Intensive Care Unit, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK
Thomas Williams & Timothy Felton
Department of Respiratory Medicine and German Centre of Lung Research (DZL), Hannover Medical School, Hannover, Germany
Tobias Welte
You can also search for this author in PubMed Google Scholar
Contributions
MF: conceptualisation, methodology, software, formal analysis, investigation, data curation, writing—original draft, visualisation, project administration. FH: conceptualisation, investigation, data curation, validation, writing—review and editing. AK, JH, RCR and TWI: data curation, validation, writing—review and editing. TWE: conceptualisation, investigation, methodology, resources, validation, writing—review and editing. TF: conceptualisation, investigation, methodology, resources, validation, writing—review and editing, supervision. AGM: conceptualisation, investigation, methodology, software, resources, validation, writing—review and editing, project administration, supervision, funding acquisition, project administration.
Corresponding author
Correspondence to Markus Fally .
Ethics declarations
Ethics approval and consent to participate.
Not applicable, as this was a methodological systematic review without patient involvement/participation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1 (docx 807 kb), search strategy, medline/pubmed.
#1: pneumonia [mh]
#2: bronchopneumonia [mh]
#3: pleuropneumonia [mh]
#4: Healthcare-Associated Pneumonia [mh]
#5: Ventilator-Associated Pneumonia [mh]
#6: pneumonia [ti]
#7: pneumonia* [ti]
#8: bronchopneumonia [ti]
#9: pleuropneumonia [ti]
#10: #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11: randomized controlled trial [pt]
#12: controlled clinical trial [pt]
#13: randomized [tiab]
#14: placebo [tiab]
#15: clinical trials as topic [mesh: noexp]
#16: randomly [tiab]
#17: trial [ti]
#18: #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
#19: animals [mh] NOT humans [mh]
#20: children [mh] NOT adults [mh]
#21: COVID-19 [mh] or (covid[ti]) or (coronavirus [ti]) or (sars-cov-2[ti]) or (covid-19[ti]) or (pandemic[ti])
#22: #19 OR #20 OR #21
#23: #18 NOT #22
#24: #10 AND #23
#25: Publication date: 2010 –2024
Cochrane library
#1: MeSH descriptor: [Pneumonia] explode all trees
#2: pneumonia*:ti
#3: #1 or #2
#4: MeSH descriptor: [COVID-19] explode all trees
#5: COVID-19:ti
#6: covid:ti
#7: coronavirus:ti
#8: sars-cov-2:ti
#9: #4 or #5 or #6 or #7 or #8
#10: #3 not #9
#11: Limit: Publication Date from 2010–2024
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fally, M., Haseeb, F., Kouta, A. et al. Unravelling the complexity of ventilator-associated pneumonia: a systematic methodological literature review of diagnostic criteria and definitions used in clinical research. Crit Care 28 , 214 (2024). https://doi.org/10.1186/s13054-024-04991-3
Download citation
Received : 28 February 2024
Accepted : 15 June 2024
Published : 02 July 2024
DOI : https://doi.org/10.1186/s13054-024-04991-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diagnostic criteria
- Inclusion criteria
- Clinical trial
- Ventilator-associated pneumonia
- Systematic review
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Emerging evidence in out-of-hospital cardiac arrest—a critical appraisal of the cardiac arrest center.

1. Introduction
3. pre-hospital care, 4. coronary care, 5. mcs and ecpr, 6. post-cardiac arrest care, 7. targeted temperature management (ttm), 8. neuroprognostication and risk assessment tools, 9. rehabilitation and post-discharge care, 10. management in cacs, 11. conclusions, author contributions, conflicts of interest.
- Berdowski, J.; Berg, R.A.; Tijssen, J.G.; Koster, R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010 , 81 , 1479–1487. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020 , 24 , 61. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sinning, C.; Ahrens, I.; Cariou, A.; Beygui, F.; Lamhaut, L.; Halvorsen, S.; Nikolaou, N.; Nolan, J.P.; Price, S.; Monsieurs, K.; et al. The cardiac arrest centre for the treatment of sudden cardiac arrest due to presumed cardiac cause—Aims, function and structure: Position paper of the Association for Acute CardioVascular Care of the European Society of Cardiology (AVCV), European Association of Percutaneous Coronary Interventions (EAPCI), European Heart Rhythm Association (EHRA), European Resuscitation Council (ERC), European Society for Emergency Medicine (EUSEM) and European Society of Intensive Care Medicine (ESICM). Eur. Heart J. Acute Cardiovasc. Care 2020 , 9 , S193–S202. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gässler, H.; Helm, M.; Hossfeld, B.; Fischer, M. Survival Following Lay Resuscitation. Dtsch. Arztebl. Int. 2020 , 117 , 871–877. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stiell, I.; Nichol, G.; Wells, G.; De Maio, V.; Nesbitt, L.; Blackburn, J.; Spaite, D. Health-Related Quality of Life Is Better for Cardiac Arrest Survivors Who Received Citizen Cardiopulmonary Resuscitation. Circulation 2003 , 108 , 1939–1944. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hasselqvist-Ax, I.; Riva, G.; Herlitz, J.; Rosenqvist, M.; Hollenberg, J.; Nordberg, P.; Ringh, M.; Jonsson, M.; Axelsson, C.; Lindqvist, J.; et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N. Engl. J. Med. 2015 , 372 , 2307–2315. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hassager, C.; Nagao, K.; Hildick-Smith, D. Out-of-hospital cardiac arrest: In-hospital intervention strategies. Lancet 2018 , 391 , 989–998. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Baekgaard, J.S.; Viereck, S.; Møller, T.P.; Ersbøll, A.K.; Lippert, F.; Folke, F. The effects of public access defibrillation on survival after out-of-hospital cardiac arrest: A systematic review of observational studies. Circulation 2017 , 137 , 954–965. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Deo, R.; Albert, C.M. Epidemiology and genetics of sudden cardiac death. Circulation 2012 , 125 , 620–637. [ Google Scholar ] [ CrossRef ]
- Dew, R.; Norton, M.; Aitken-Fell, P.; Blance, P.; Miles, S.; Potts, S.; Wilkes, S. Knowledge and barriers of out of hospital cardiac arrest bystander intervention and public access automated external defibrillator use in the Northeast of England: A cross-sectional survey study. Intern. Emerg. Med. 2024 , 1–11. [ Google Scholar ] [ CrossRef ]
- Berglund, E.; Hollenberg, J.; Jonsson, M.; Svensson, L.; Claesson, A.; Nord, A.; Nordberg, P.; Forsberg, S.; Rosenqvist, M.; Lundgren, P.; et al. Effect of Smartphone Dispatch of Volunteer Responders on Automated External Defibrillators and Out-of-Hospital Cardiac Arrests: The SAMBA Randomized Clinical Trial. JAMA Cardiol. 2023 , 8 , 81–88. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lapidus, O.; Jonsson, M.; Svensson, L.; Hollenberg, J.; Berglund, E.; Riva, G.; Claesson, A.; Nordberg, P.; Rosenqvist, M.; Forsberg, S.; et al. Effects of a volunteer responder system for out-of-hospital cardiac arrest in areas of different population density—A retrospective cohort study. Resuscitation 2023 , 191. [ Google Scholar ] [ CrossRef ]
- Morin, F.; Douillet, D.; Lamhaut, L.; Fadel, M.; Savary, D. Deployment of “super lay-rescuers” equipped with AED to improve OHCA survival: An innovative partnership between emergency medical service, city hall and a mobile application in France. Resuscitation 2022 , 176 , 51–52. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Morin, F.; Douillet, D.; Sokpoh, E.; Lamhaut, L.; Savary, D. One year after implementation of «super lay-rescuers» equipped with AED to improve survival of OHCA. Resuscitation 2024 , 197 , 110147. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schierbeck, S.; Hollenberg, J.; Nord, A.; Svensson, L.; Nordberg, P.; Ringh, M.; Forsberg, S.; Lundgren, P.; Axelsson, C.; Claesson, A. Automated external defibrillators delivered by drones to patients with suspected out-of-hospital cardiac arrest. Eur. Heart J. 2022 , 43 , 1478–1487. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, X.; Yuan, Q.; Wang, G.; Bian, Y.; Xu, F.; Chen, Y. Drones delivering automated external defibrillators: A new strategy to improve the prognosis of out-of-hospital cardiac arrest. Resuscitation 2022 , 182 , 109669. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schierbeck, S.; Nord, A.; Svensson, L.; Ringh, M.; Nordberg, P.; Hollenberg, J.; Lundgren, P.; Folke, F.; Jonsson, M.; Forsberg, S.; et al. Drone delivery of automated external defibrillators compared with ambulance arrival in real-life suspected out-of-hospital cardiac arrests: A prospective observational study in Sweden. Lancet Digit. Health 2023 , 5 , e862–e871. [ Google Scholar ] [ CrossRef ]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023 , 44 , 3720–3826. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lotfi, A.; Klein, L.W.; Hira, R.S.; Mallidi, J.; Mehran, R.; Messenger, J.C.; Pinto, D.S.; Mooney, M.R.; Rab, T.; Yannopoulos, D.; et al. SCAI expert consensus statement on out of hospital cardiac arrest. Catheter. Cardiovasc. Interv. 2020 , 96 , 844–861. [ Google Scholar ] [ CrossRef ]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013 , 61 , e78–e140. [ Google Scholar ] [ CrossRef ]
- Kern, K.B.; Lotun, K.; Patel, N.; Mooney, M.R.; Hollenbeck, R.D.; McPherson, J.A.; McMullan, P.W.; Unger, B.; Hsu, C.-H.; Seder, D.B.; et al. Outcomes of Comatose Cardiac Arrest Survivors with and without ST-Segment Elevation Myocardial Infarction: Importance of Coronary Angiography. JACC Cardiovasc. Interv. 2015 , 8 , 1031–1040. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van De Werf, F. The history of coronary reperfusion. Eur. Heart J. 2014 , 35 , 2510–2515. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kashef, M.A.; Kashef, M.A.; Lotfi, A.S.; Lotfi, A.S. Evidence-Based Approach to Out-of-Hospital Cardiac Arrest. Curr. Treat. Options Cardiovasc. Med. 2021 , 23 , 1–14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Meaney, P.A.; Nadkarni, V.M.; Kern, K.B.; Indik, J.H.; Halperin, H.R.; Berg, R.A. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit. Care Med. 2010 , 38 , 101–108. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Han, K.S.; Lee, S.W.; Lee, E.J.; Kwak, M.H.; Kim, S.J. Association between shockable rhythm conversion and outcomes in patients with out-of-hospital cardiac arrest and initial non-shockable rhythm, according to the cause of cardiac arrest. Resuscitation 2019 , 142 , 144–152. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, J.G.; Shin, H.; Cho, J.H.; Choi, H.Y.; Kim, W.; Kim, J.; Moon, S.; Ahn, C.; Lee, J.; Cho, Y.; et al. Prognostic value of changes in the cardiac arrest rhythms from the prehospital stage to the emergency department in out-of-hospital cardiac arrest patients without prehospital returns of spontaneous circulation: A nationwide observational study. PLoS ONE 2021 , 16 , e0257883. [ Google Scholar ] [ CrossRef ]
- Cournoyer, A.; Cossette, S.; Potter, B.J.; Daoust, R.; de Montigny, L.; Londei-Leduc, L.; Lamarche, Y.; Ross, D.; Morris, J.; Chauny, J.-M.; et al. Prognostic impact of the conversion to a shockable rhythm from a non-shockable rhythm for patients suffering from out-of-hospital cardiac arrest. Resuscitation 2019 , 140 , 43–49. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J. Cardiovasc. Imaging 2023 , 24 , e86–e88. [ Google Scholar ] [ CrossRef ]
- Hussein, L.; Rehman, M.A.; Jelic, T.; Berdnikov, A.; Teran, F.; Richards, S.; Askin, N.; Jarman, R. Transoesophageal echocardiography in cardiac arrest: A systematic review. Resuscitation 2021 , 168 , 167–175. [ Google Scholar ] [ CrossRef ]
- Teran, F.; Dean, A.J.; Centeno, C.; Panebianco, N.L.; Zeidan, A.J.; Chan, W.; Abella, B.S. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation 2019 , 137 , 140–147. [ Google Scholar ] [ CrossRef ]
- Lemkes, J.S.; Janssens, G.N.; Van Der Hoeven, N.W.; Jewbali, L.S.D.; Dubois, E.A.; Meuwissen, M.M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography After Cardiac Arrest Without ST Segment Elevation: One-Year Outcomes of the COACT Randomized Clinical Trial. JAMA Cardiol. 2020 , 5 , 1358–1365. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2021 , 385 , 2544–2553. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hauw-Berlemont, C.; Lamhaut, L.; Diehl, J.L.; Andreotti, C.; Varenne, O.; Leroux, P.; Lascarrou, J.-B.; Guerin, P.; Loeb, T.; Roupie, E.; et al. Emergency vs Delayed Coronary Angiogram in Survivors of Out-of-Hospital Cardiac Arrest: Results of the Randomized, Multicentric EMERGE Trial. JAMA Cardiol. 2022 , 7 , 700–707. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Coronary Angiography After Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation: One-Year Outcomes of a Randomized Clinical Trial. JAMA Cardiol. 2023 , 8 , 827–834. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hamidi, F.; Anwari, E.; Spaulding, C.; Hauw-Berlemont, C.; Vilfaillot, A.; Viana-Tejedor, A.; Kern, K.B.; Hsu, C.-H.; Bergmark, B.A.; Qamar, A.; et al. Early versus delayed coronary angiography in patients with out-of-hospital cardiac arrest and no ST-segment elevation: A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Cardiol. 2023 , 113 , 561–569. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Verma, B.R.; Sharma, V.; Shekhar, S.; Kaur, M.; Khubber, S.; Bansal, A.; Singh, J.; Ahuja, K.R.; Nazir, S.; Chetrit, M.; et al. Coronary Angiography in Patients with Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2020 , 13 , 2193–2205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Franco, D.; Goslar, T.; Radsel, P.; De Luca, N.; Esposito, G.; Izzo, R.; Tesorio, T.; Barbato, E.; Noc, M. Coronary features across the spectrum of out-of-hospital cardiac arrest with ST-elevation myocardial infarction (CAD-OHCA study). Resuscitation 2023 , 193 , 109981. [ Google Scholar ] [ CrossRef ]
- Stähli, B.E.; Varbella, F.; Linke, A.; Schwarz, B.; Felix, S.B.; Seiffert, M.; Kesterke, R.; Nordbeck, P.; Witzenbichler, B.; Lang, I.M.; et al. Timing of Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2023 , 389 , 1368–1379. [ Google Scholar ] [ CrossRef ]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; De Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017 , 377 , 2419–2432. [ Google Scholar ] [ CrossRef ]
- Thiele, H.; Akin, I.; Sandri, M.; De Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N. Engl. J. Med. 2018 , 379 , 1699–1710. [ Google Scholar ] [ CrossRef ]
- Kolte, D.; Sardar, P.; Khera, S.; Zeymer, U.; Thiele, H.; Hochadel, M.; Radovanovic, D.; Erne, P.; Hambraeus, K.; James, S.; et al. Culprit Vessel-Only Versus Multivessel Percutaneous Coronary Intervention in Patients with Cardiogenic Shock Complicating ST-Segment-Elevation Myocardial Infarction: A Collaborative Meta-Analysis. Circ. Cardiovasc. Interv. 2017 , 10 , e005582. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abrams, D.; MacLaren, G.; Lorusso, R.; Price, S.; Yannopoulos, D.; Vercaemst, L.; Bělohlávek, J.; Taccone, F.S.; Aissaoui, N.; Shekar, K.; et al. Extracorporeal cardiopulmonary resuscitation in adults: Evidence and implications. Intensiv. Care Med. 2021 , 48 , 1–15. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Becher, P.M.; Schrage, B.; Sinning, C.R.; Schmack, B.; Fluschnik, N.; Schwarzl, M.; Waldeyer, C.; Lindner, D.; Seiffert, M.; Neumann, J.T.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Cardiopulmonary Support. Circulation 2018 , 138 , 2298–2300. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lüsebrink, E.; Kellnar, A.; Krieg, K.; Binzenhöfer, L.; Scherer, C.; Zimmer, S.; Schrage, B.; Fichtner, S.; Petzold, T.; Braun, D.; et al. Percutaneous Transvalvular Microaxial Flow Pump Support in Cardiology. Circulation 2022 , 145 , 1254–1284. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gillon, S.; Zheng, C.; Feng, Z.; Fleig, M.; Scquizzato, T.; Belohlavek, J.; Lorusso, R.; Lone, N.; Swol, J. GEospatial aNalysis of ExtRacorporeal membrane oxygenATion in Europe (GENERATE). Perfusion 2023 , 38 , 24–39. [ Google Scholar ] [ CrossRef ]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023 , 389 , 1286–1297. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zeymer, U.; Freund, A.; Hochadel, M.; Ostadal, P.; Belohlavek, J.; Rokyta, R.; Massberg, S.; Brunner, S.; Lüsebrink, E.; Flather, M.; et al. Venoarterial extracorporeal membrane oxygenation in patients with infarct-related cardiogenic shock: An individual patient data meta-analysis of randomised trials. Lancet 2023 , 402 , 1338–1346. [ Google Scholar ] [ CrossRef ]
- Becher, P.M.; Goßling, A.; Schrage, B.; Twerenbold, R.; Fluschnik, N.; Seiffert, M.; Bernhardt, A.M.; Reichenspurner, H.; Blankenberg, S.; Westermann, D. Procedural volume and outcomes in patients undergoing VA-ECMO support. Crit. Care 2020 , 24 , 1–10. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schrage, B.; Sundermeyer, J.; Blankenberg, S.; Colson, P.; Eckner, D.; Eden, M.; Eitel, I.; Frank, D.; Frey, N.; Graf, T.; et al. Timing of Active Left Ventricular Unloading in Patients on Venoarterial Extracorporeal Membrane Oxygenation Therapy. JACC Heart Fail. 2023 , 11 , 321–330. [ Google Scholar ] [ CrossRef ]
- Kim, M.C.; Lim, Y.; Lee, S.H.; Shin, Y.; Ahn, J.H.; Hyun, D.Y.; Cho, K.H.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; et al. Early left ventricular unloading after extracorporeal membrane oxygenation: Rationale and design of EARLY-UNLOAD trial. ESC Heart Fail. 2023 , 10 , 2672–2679. [ Google Scholar ] [ CrossRef ]
- Study Details | Left Ventricular Unloading to Improve Outcome in Cardiogenic Shock Patients on VA-ECMO | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05577195 (accessed on 20 April 2024).
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024 , 390 , 1382–1393. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hutin, A.; Abu-Habsa, M.; Burns, B.; Bernard, S.; Bellezzo, J.; Shinar, Z.; Torres, E.C.; Gueugniaud, P.-Y.; Carli, P.; Lamhaut, L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation 2018 , 130 , 44–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, Y.-S.; Lin, J.-W.; Yu, H.-Y.; Ko, W.-J.; Jerng, J.-S.; Chang, W.-T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008 , 372 , 554–561. [ Google Scholar ] [ CrossRef ]
- Wengenmayer, T.; Rombach, S.; Ramshorn, F.; Biever, P.; Bode, C.; Duerschmied, D.; Staudacher, D.L. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit. Care 2017 , 21 , 1–6. [ Google Scholar ] [ CrossRef ]
- Suverein, M.M.; Delnoij, T.S.; Lorusso, R.; Bruinsma, G.J.B.B.; Otterspoor, L.; Kraemer, C.V.E.; Vlaar, A.P.; van der Heijden, J.J.; Scholten, E.; Uil, C.D.; et al. Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2023 , 388 , 299–309. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022 , 327 , 737–747. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gonzalez, D.; Dahiya, G.; Mutirangura, P.; Ergando, T.; Mello, G.; Singh, R.; Bentho, O.; Elliott, A.M. Post Cardiac Arrest Care in the Cardiac Intensive Care Unit. Curr. Cardiol. Rep. 2024 , 26 , 35–49. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021 , 47 , 369. [ Google Scholar ] [ CrossRef ]
- Hirsch, K.G.; Abella, B.S.; Amorim, E.; Bader, M.K.; Barletta, J.F.; Berg, K.; Callaway, C.W.; Friberg, H.; Gilmore, E.J.; Greer, D.M.; et al. Critical Care Management of Patients After Cardiac Arrest: A Scientific Statement from the American Heart Association and Neurocritical Care Society. Circulation 2024 , 149 , e168. [ Google Scholar ] [ CrossRef ]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002 , 346 , 549–556. [ Google Scholar ] [ CrossRef ]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. N. Engl. J. Med. 2002 , 346 , 557–563. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peberdy, M.A.; Callaway, C.W.; Neumar, R.W.; Geocadin, R.G.; Zimmerman, J.L.; Donnino, M.; Gabrielli, A.; Silvers, S.M.; Zaritsky, A.L.; Merchant, R.; et al. Part 9: Post–Cardiac Arrest Care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010 , 122 (Suppl. S3), S768–S786. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted Temperature Management at 33 °C versus 36 °C after Cardiac Arrest. N. Engl. J. Med. 2013 , 369 , 2197–2206. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021 , 384 , 2283–2294. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Granfeldt, A.; Holmberg, M.J.; Nolan, J.P.; Soar, J.; Andersen, L.W. Targeted temperature management in adult cardiac arrest: Systematic review and meta-analysis. Resuscitation 2021 , 167 , 160–172. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Holgersson, J.; Meyer, M.A.S.; Dankiewicz, J.; Lilja, G.; Ullén, S.; Hassager, C.; Cronberg, T.; Wise, M.P.; Bělohlávek, J.; Hovdenes, J.; et al. Hypothermic versus Normothermic Temperature Control after Cardiac Arrest. NEJM Evid. 2022 , 1 , EVIDoa2200137. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post–Cardiac Arrest Care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015 , 132 (Suppl. S2), S465–S482. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Perman, S.M.; Bartos, J.A.; Marina, F.; Rios, D.; Donnino, M.W.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Maciel, C.B.; Menon, V.; et al. Temperature Management for Comatose Adult Survivors of Cardiac Arrest: A Science Advisory From the American Heart Association. Circulation 2023 , 148 , 982–988. [ Google Scholar ] [ CrossRef ]
- Sandroni, C.; Nolan, J.P.; Andersen, L.W.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Lilja, G.; Morley, P.T.; et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Intensive Care Med. 2022 , 48 , 261–269. [ Google Scholar ] [ CrossRef ]
- Rajajee, V.; Muehlschlegel, S.; Wartenberg, K.E.; Alexander, S.A.; Busl, K.M.; Chou, S.H.Y.; Creutzfeldt, C.J.; Fontaine, G.V.; Fried, H.; Hocker, S.E.; et al. Guidelines for Neuroprognostication in Comatose Adult Survivors of Cardiac Arrest. Neurocriti. Care 2023 , 38 , 533–563. [ Google Scholar ] [ CrossRef ]
- Dragancea, I.; Wise, M.P.; Al-Subaie, N.; Cranshaw, J.; Friberg, H.; Glover, G.; Pellis, T.; Rylance, R.; Walden, A.; Nielsen, N.; et al. Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation 2017 , 117 , 50–57. [ Google Scholar ] [ CrossRef ]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.-L.; Geri, G.; Perier, M.-C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2016 , 37 , 3222–3228. [ Google Scholar ] [ CrossRef ]
- Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; Thuong, M.; Monchi, M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: The OHCA score. Eur. Heart J. 2006 , 27 , 2840–2845. [ Google Scholar ] [ CrossRef ]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Undén, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 2017 , 21 , 96. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sunderland, N.; Cheese, F.; Leadbetter, Z.; Joshi, N.V.; Mariathas, M.; Felekos, I.; Biswas, S.; Dalton, G.; Dastidar, A.; Aziz, S.; et al. Validation of the MIRACLE2 Score for Prognostication After Out-of-hospital Cardiac Arrest. Interv. Cardiol. Rev. 2023 , 18 , e29. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pareek, N.; Kordis, P.; Beckley-Hoelscher, N.; Pimenta, D.; Kocjancic, S.T.; Jazbec, A.; Nevett, J.; Fothergill, R.; Kalra, S.; Lockie, T.; et al. A practical risk score for early prediction of neurological outcome after out-of-hospital cardiac arrest: MIRACLE2. Eur. Heart J. 2020 , 41 , 4508–4517. [ Google Scholar ] [ CrossRef ]
- Schmidbauer, S.; Rylander, C.; Cariou, A.; Wise, M.P.; Thomas, M.; Keeble, T.R.; Erlinge, D.; Haenggi, M.; Wendel-Garcia, P.D.; Bělohlávek, J.; et al. Comparison of four clinical risk scores in comatose patients after out-of-hospital cardiac arrest. Resuscitation 2023 , 191 , 109949. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pek, P.P.; Fan, K.C.; Ong, M.E.H.; Luo, N.; Østbye, T.; Lim, S.L.; Ho, A.F. Determinants of health-related quality of life after out-of-hospital cardiac arrest (OHCA): A systematic review. Resuscitation 2023 , 188 , 109794. [ Google Scholar ] [ CrossRef ]
- Yeo, J.W.; Ng, Z.H.C.; Goh, A.X.C.; Gao, J.F.; Liu, N.; Lam, S.W.S.; Chia, Y.W.; Perkins, G.D.; Ong, M.E.H.; Ho, A.F.W.; et al. Impact of Cardiac Arrest Centers on the Survival of Patients with Nontraumatic Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022 , 11 , e023806. [ Google Scholar ] [ CrossRef ]
- Xin Chun Goh, A.; Cong Seow, J.; Yong Hao Lai, M.; Liu, N.; Man Goh, Y.; Eng Hock Ong, M.; Lim, S.L.; Ho, J.S.Y.; Yeo, J.W.; Ho, A.F.W.; et al. Association of High-Volume Centers with Survival Outcomes Among Patients with Nontraumatic Out-of-Hospital Cardiac Arrest A Systematic Review and Meta-Analysis + Supplemental content. JAMA Netw. Open 2022 , 5 , 2214639. [ Google Scholar ]
- Schober, A.; Sterz, F.; Laggner, A.N.; Poppe, M.; Sulzgruber, P.; Lobmeyr, E.; Datler, P.; Keferböck, M.; Zeiner, S.; Nuernberger, A.; et al. Admission of out-of-hospital cardiac arrest victims to a high volume cardiac arrest center is linked to improved outcome. Resuscitation 2016 , 106 , 42–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chien, C.Y.; Tsai, S.L.; Tsai, L.H.; Chen, C.B.; Seak, C.J.; Weng, Y.M.; Lin, C.-C.; Ng, C.-J.; Chien, W.-C.; Huang, C.-H.; et al. Impact of Transport Time and Cardiac Arrest Centers on the Neurological Outcome After Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Study. J. Am. Heart Assoc. 2020 , 9 , 15544. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jung, E.; Ro, Y.S.; Park, J.H.; Ryu, H.H.; Do, S.S. Clinical Medicine Direct Transport to Cardiac Arrest Center and Survival Outcomes after Out-of-Hospital Cardiac Arrest by Urbanization Level. J. Clin. Med. 2022 , 2022 , 1033. [ Google Scholar ] [ CrossRef ]
- von Vopelius-Feldt, J.; Perkins, G.D.; Benger, J. Association between admission to a cardiac arrest centre and survival to hospital discharge for adults following out-of-hospital cardiac arrest: A multi-centre observational study. Resuscitation 2021 , 160 , 118–125. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yeung, J.; Matsuyama, T.; Bray, J.; Reynolds, J.; Skrifvars, M.B. Does care at a cardiac arrest centre improve outcome after out-of-hospital cardiac arrest?—A systematic review. Resuscitation 2019 , 137 , 102–115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Elmer, J.; Rittenberger, J.C.; Coppler, P.J.; Guyette, F.; Doshi, A.A.; Callaway, C.W.; Pittsburgh Post-Cardiac Arrest Service. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation 2016 , 108 , 48–53. [ Google Scholar ] [ CrossRef ]
- 88. May, T.L.; Lary, C.W.; Riker, R.R.; Friberg, H.; Patel, N.; Søreide, E.; McPherson, J.A.; Undén, J.; Hand, R.; Sunde, K.; et al. Variability in functional outcome and treatment practices by treatment center after out-of-hospital cardiac arrest: Analysis of International Cardiac Arrest Registry. Intensive Care Med. 2019 , 45 , 637–646. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patterson, T.; Perkins, G.D.; Perkins, A.; Clayton, T.; Evans, R.; Dodd, M.; Robertson, S.; Wilson, K.; Mellett-Smith, A.; Fothergill, R.T.; et al. Expedited transfer to a cardiac arrest centre for non-ST-elevation out-of-hospital cardiac arrest (ARREST): A UK prospective, multicentre, parallel, randomised clinical trial. Lancet 2023 , 402 , 1329–1337. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Greif, R.; Bhanji, F.; Bigham, B.L.; Bray, J.; Breckwoldt, J.; Cheng, A.; Duff, J.P.; Gilfoyle, E.; Hsieh, M.-J.; Iwami, T.; et al. Education, Implementation, and Teams: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020 , 142 , S222–S283. [ Google Scholar ] [ CrossRef ]
- Berg, K.M.; Bray, J.E.; Ng, K.C.; Liley, H.G.; Greif, R.; Carlson, J.N.; Morley, P.T.; Drennan, I.R.; Smyth, M.; Scholefield, B.R.; et al. 2023 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations: Summary from the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Resuscitation 2024 , 195 , e187–e280. [ Google Scholar ] [ CrossRef ]
- Rott, N.; Scholz, K.; Busch, H.; Frey, N.; Kelm, M.; Thiele, H.; Böttiger, B. Cardiac Arrest Center Certification for out-of-hospital cardiac arrest patients successfully established in Germany. Resuscitation 2020 , 156 , 1–3. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Voß, F.; Thevathasan, T.; Scholz, K.H.; Böttiger, B.W.; Scheiber, D.; Kabiri, P.; Bernhard, M.; Kienbaum, P.; Jung, C.; Westenfeld, R.; et al. Accredited cardiac arrest centers facilitate eCPR and improve neurological outcome. Resuscitation 2024 , 194 , 110069. [ Google Scholar ] [ CrossRef ]
- Rott, N.; Böttiger, B. Five years of Cardiac Arrest Center (CAC) certification in Germany—A success story. Resuscitation 2024 , 196 , 110130. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rott, N.; Wingen, S.; Müller, D.; Böttiger, B.W. Cardiac arrest centers—Certification fosters inflow of patients by emergency medical services. Med. Klin.-Intensiv. Notfallmedizin 2022 , 118 , 263–268. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grübl, T.; Nauheimer, D.; Wolff, H.; Gehret, G.; Rott, N.; Schmidbauer, W.; Schieffer, B.; Gliwitzky, B. Zertifizierung von Cardiac-Arrest-Zentren. Notf.+ Rettungsmedizin 2022 , 26 , 23–29. [ Google Scholar ] [ CrossRef ]
- Pöss, J.; Sinning, C.; Schreiner, I.; Apfelbacher, C.; Drewitz, K.-P.; Hösler, N.; Schneider, S.; Pieske, B.; Böttiger, B.W.; Ewen, S.; et al. German Cardiac Arrest Registry: Rationale and design of G-CAR. Clin. Res. Cardiol. 2022 , 112 , 455–463. [ Google Scholar ] [ CrossRef ]
| Authors | Type of Evidence | Total n° of Patients n | N° of Patients Treated in CAC vs. Non-CAC | Primary Endpoints/ Outcome Measures | % of Coronary Angiographies | Key Outcomes Regarding CACs |
|---|---|---|---|---|---|---|
| ] | Randomized controlled trial | 862 | CAC: 431 (50%) vs. Non-CAC: 431 (50%), 411 vs. 412 included in analysis | All-cause mortality at 30 days | CAC: 231 of 412 (56%), non-CAC: 153 of 410 (37%) | No significant difference, p = 0.96 (95% CI 0.9–1.11) |
| ] | Systematic review/ meta-analysis | 147.943 (36 studies) | n.a. | (a) Survival to hospital discharge or 30 days (b) Survival to hospital discharge or 30 days with favorable neurological outcome | n.a. | (a) CAC favorable, adjusted OR 1.92 (95% CI 1.59–2.32) (b) CAC favorable, adjusted OR 1.85 (95% CI 1.52–2.26) |
| ] | Systematic review/ meta-analysis | 82.769 (16 studies) | n.a. | (a) Survival to hospital discharge or 30 days (b) Neurological outcomes at hospital discharge or 30 days | n.a. | (a) CAC favorable, adjusted OR 1.28 (95% CI 1.00–1.64) (b) No significant difference, adjusted OR 0.96 (95% CI 0.77–1.20) |
| ] | Observational | 95.931 | CAC: 23.292 (24.3%) vs. Non-CAC: 72.639 (75.7%) | (a) Survival to hospital discharge (b) Good neurologic recovery | n.a. | (a) CAC favorable, adjusted OR 1.70 (1.60–1.80) (b) CAC favorable, adjusted OR 1.75 (1.63–1.89) |
| ] | Observational | 10.650 (4.368 after propensity score matching) | 24/7 PPCI center: 5.375 (50.5%) vs. Other hospitals: 5.275 (49.5%) High volume center: 5.216 (49.0%) vs. Other hospitals: 5.434 (51.0%) | Survival to hospital discharge | n.a. | 24/7 PPCI centers favorable, adjusted OR 1.69 (95% CI 1.28 to 2.23) High volume centers favorable, adjusted OR 1.41 (95% CI 1.14 to 1.75) |
| ] | Observational | 6.655 (5.156 after propensity score matching) | CAC: 4.039 (60.7%) vs. Non-CAC: 2.616 (39.3%) | (a) (Association of transport time with) survival to hospital discharge (b) (Association of transport time with) good neurological outcome at discharge | n.a. | (a) CAC favorable in shockable rhythms, adjusted OR 2.20 (95% CI 1.29–3.75) for transport time < 8 min and adjusted OR 1.92 (95% CI 1.25–2.94) for transport time ≥ 8 min (b) CAC favorable in shockable rhythms, adjusted OR 2.7 (95% CI 1.4–5.22) for transport time < 8 min and adjusted OR 1.92 (95% CI 1.25–2.94) for transport time ≥ 8 min |
| ] | Observational | 3.855 | High-performing centers: 873 vs. Low-performing centers: 1.311 | CPC (Cerebral Performance Category) score at hospital discharge | Unconscious catheterization: High-performing centers 451 (53%) vs. Low-performing centers: 411 (32%) | Center specific risk standardized rates for good functional outcome range from 0.47 (0.37–0.58) to 0.2 (0.12–0.26) |
| ] | Systematic review/meta-analysis | Endpoint (a): 46.164 Endpoint (b): 30.080 (17 studies) | (a) CAC: 18.449 vs. Other hospitals: 27.507 (b) CAC: 3.086 vs. Other hospitals: 587 | (a) Survival to 30 days with favorable neurological outcome (b) Survival to hospital discharge with favorable neurological outcome | n.a. | (a) No significant difference (OR 2.92, 95% CI 0.68–12.48) (b) CACs favorable (OR 2.22, 95% CI 1.74–2.84) |
| ] | Observational | 2.238 | High-volume center (>100 cases/year): 378 vs. Medium- and low-volume centers: 483 | Survival to 30 days with favorable neurological outcome (CPC 1 or 2) | n.a. | High frequency centers favorable (OR 5.2, 95% CI 1.2–21.7, p = 0.025) |
| ] | Observational | 987 | High-volume center: 680 vs. Medium- and low-volume centers: 307 | Predictors of long-term survival | High-volume center: 264 of 680 (39%) vs. Medium- and low-volume centers: 115 of 307 (37%) | High-volume center favorable, adjusted HR for treatment in medium- and low-volume centers: 1.58 (95% CI 1.27–1.95), p < 0.001 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Memenga, F.; Sinning, C. Emerging Evidence in Out-of-Hospital Cardiac Arrest—A Critical Appraisal of the Cardiac Arrest Center. J. Clin. Med. 2024 , 13 , 3973. https://doi.org/10.3390/jcm13133973
Memenga F, Sinning C. Emerging Evidence in Out-of-Hospital Cardiac Arrest—A Critical Appraisal of the Cardiac Arrest Center. Journal of Clinical Medicine . 2024; 13(13):3973. https://doi.org/10.3390/jcm13133973
Memenga, Felix, and Christoph Sinning. 2024. "Emerging Evidence in Out-of-Hospital Cardiac Arrest—A Critical Appraisal of the Cardiac Arrest Center" Journal of Clinical Medicine 13, no. 13: 3973. https://doi.org/10.3390/jcm13133973
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
SYSTEMATIC REVIEW article
The impact of arts on prescription on individual health and wellbeing: a systematic review with meta-analysis.

- 1 Clinical Research Center, Social Medicine and Health Policy, Department of Clinical Science and Centre for Primary Health Care, Lund University and Region Skåne, Malmö, Sweden
- 2 National Competence Center for Culture, Health and Care, Nord Universitet, Levanger, Norway
- 3 Department of Health and Social Sciences, University of the West of England, Bristol, United Kingdom
- 4 General Internal Medicine, St Luke’s International Hospital, Tokyo, Japan
- 5 Faculty of Health, Education, Medicine, Social Care, and Education, School of Allied and Public Health, Anglia Ruskin University, Chelmsford, United Kingdom
Introduction: The evidence-base for the impact of participating in the arts for different aspects of health and wellbeing is growing. Arts on Prescription has gained increasing recognition as a method for fostering connections among individuals and communities, however, to date no systematic review of the impact on individual health and wellbeing has been conducted. This review aims to provide an understanding of individual health and wellbeing outcomes from participation in Arts on Prescription programmes.
Methods: Major electronic databases were systematically searched, including Cochrane Library; Web of Science; ProQuest; CINAHL; Arts & Humanities; Ebsocohost; Pubmed; PsycINFO. Other databases were also used: Google Scholar and websites of specific organizations, e.g., NHS Evidence, Kings Fund, Health foundation, Nuffield Trust and NESTA and University of Florida Arts-in Medicine Repository. The review used PRISMA reporting structures. Critical Appraisal Skills Programme (CASP) templates were used for qualitative and quantitative studies, and the Mixed Methods Appraisal Tool (MMAT) for studies with a mixed methods protocol to assess quality and risk of bias. A narrative review of the qualitative data was conducted. For quantitative outcomes, a meta-analysis for studies that met inclusion criteria was conducted, and a narrative review made of secondary and heterogeneous outcomes and approaches.
Results: 7,805 records were identified but only 25 records were included as studies with a focus on the impact on individual health and wellbeing. Studies were conducted in Australia, Denmark, Sweden, United Kingdom, and the United States. Programmes were held in community settings, arts venues, GP surgeries, primary healthcare settings, and one school. Most interventions varied from 8 to 10 weeks and included a wide range of different arts activities. Qualitative themes included social benefits, psychological benefits and progression opportunities. The meta-analysis showed a statistically significant improvement in wellbeing, and the narrative review identified promising outcomes that require further evidential support (e.g., reductions in anxiety and depression).
Discussion: Arts on Prescription programmes are an appropriate intervention for improving psychosocial wellbeing, providing both social and psychological benefits as well as progression opportunities. We discuss the various qualitative and quantitative outcomes, along with potential ‘active ingredients’ and barriers to participation (physical, psychological and social).
Systematic review registration: PROSPERO, identifier CRD42023408974, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023408974 .
Introduction
In several countries across the continents, Arts on Prescription (AoP) [also referred to as Arts on Referral (AoR)] has gained increasing recognition as a method for fostering connections among individuals and communities through creative activities, with the overarching goal of enhancing health and overall wellbeing ( 1 – 4 ). Arts on Prescription falls within the broader framework of social prescribing (SP), a mechanism through which primary healthcare practitioners, including General Practitioners (GPs) and healthcare professionals, can proactively refer service users to support with housing, befriending services and community-based initiatives, encompassing activities such as horticulture, culinary arts, communal walking, creative pursuits, or various other group engagements ( 5 – 7 ). Social prescribing has been described as a complex process, rather than an intervention, with different stages and interactions across the referral pathway ( 8 ). A number of definitions of social prescribing have been proposed, but for the purposes of this review the following is adopted; ‘a holistic, person-centred and community-based approach to health and wellbeing that bridges the gap between clinical and non-clinical supports and services’ ( 8 , p. 7). In the UK there are a number of different models of social prescribing delivery in operation ( 9 ). In essence, though, social prescribing enables a clinical professional to refer a person to a link worker, or community connector who then connects the person to non-clinical supports and services in the community. The premise underlying this referral process is the expectation that engagement with activities will contribute to the enhancement of psychosocial wellbeing ( 7 , 10 ).
Research to build an evidence base for this premise, and the mechanisms through which it occurs, has been accruing ( 11 – 13 ). However, it has proven difficult to integrate results since definitions and metrics to explore conceptual linkages between social prescribing and system/community outcomes are not standardized ( 14 ). Further, different methods of delivery and implementation of social prescribing present challenges for interpretation of outcomes and mechanisms ( 7 , 10 ). Nevertheless, research on Social Prescribing ( 15 ), and Arts on Prescription specifically, has increased in recent years as demonstrated in this review, such that attempts to integrate findings through systematic reviews are necessary to synthesize the collective evidence base and provide insights for both future research agendas and good practice for the delivery of programmes.
There is increasing endorsement of Social Prescribing initiatives by both policy-making bodies and governments, with the expectation that Social Prescribing can help to reduce the financial burden of health care, by addressing social determinants of health, reducing loneliness and the impact of health inequalities ( 16 – 21 ). This systematic review assumes a timely relevance, seeking to comprehensively evaluate the extant body of evidence dedicated to Arts on Prescription, especially useful since there are no prior systematic reviews on this topic.
In this systematic review, we include Arts on Prescription programmes that involve a range of different arts activities, and/or events, where groups of participants engage with different types of arts depending on the context (e.g., visual arts, creative writing, dance, or music). Programmes vary in delivery, in terms of length, numbers of participants and art activities offered ( 4 , 22 ). Additionally, Arts on Prescription programmes differ in that they are designed to serve diverse demographic groups and are implemented in a multitude of settings ( 1 , 3 ). Yet, they have in common a referral being made to engage with a programme of art activities over a period of several weeks, with the expectation that this will improve psychosocial wellbeing. It is important to note that the Arts on Prescription model differs from creative arts therapies and from art classes. The focus is on process, play and social community rather than skill development, and art is not used to facilitate psychotherapy. The facilitators are not trained therapists/psychotherapists, and are positioned as ‘arts for health’ facilitators rather than ‘art teachers’ ( 10 ).
The primary objective of this systematic review is to clarify and critically evaluate outcomes and wider impact of community-based Arts on Prescription and Arts on Referral programmes on individual health and wellbeing. The research protocol was registered with PROSPERO (2023 CRD42023408974) ( 23 ).
Methods of searching the literature, the inclusion and exclusion criteria and methods of quality assessment were determined and specified in advance in a protocol which was registered with PROSPERO, as stated above. In the development of this protocol the Preferred Reporting Items for Systematic Reviews (PRISMA) reporting structure systematic reviews was followed ( 24 ). The inclusion and exclusion criteria were developed through PICOC and SPICE frameworks ( 25 ). Quality and risk of bias appraisals were planned to be made with the Critical Appraisal Skills Programme (CASP) templates and the Mixed Methods Appraisal Tool (MMAT) ( 26 , 27 ). Data extraction followed guidance from the Centre for Reviews and Dissemination ( 25 ) and Cochrane Handbook for Systematic Reviews of Interventions ( 28 ). All procedures and processes were piloted and checked for accuracy independently by two authors, with any discrepancies resolved through a third author, throughout all stages of the review process, as detailed below.
Literature search
A systematic search was conducted using the following keywords: “art on prescription” OR “art on referral” OR (art on prescription) OR (art on referral) OR (arts on prescription) OR (“arts on prescription”) OR (arts on referral) OR (“arts on referral”) OR (culture on prescription) OR (“culture on prescription”) OR (culture on referral) OR (“culture on referral”). Keywords were carefully chosen to provide comprehensive coverage of the use of the arts in social prescribing based on preliminary analyses of terms used in the literature and consultation with experts. The focus was on ‘arts prescribing’ rather than broader forms of arts interventions, because this term is widely known and acts as an umbrella term for an array of different arts activities, in the same way that the term ‘social prescribing’ represents a wide range of different activities and has been used in systematic reviews of social prescribing [see for example ( 29 )]. Phrase searches were used with and without inverted commas in order to find both exact searches and publications with variations of terminology. Both the titles and abstract fields were searched to maximize sensitivity. Where possible, the language of articles was set to “English” and dates from 1994 to 2023. This search strategy was developed and tested by the research team using databases Web of Science and PubMed in March 2023. After careful formulation, searches were conducted between April 2023 and July 2023 using the following databases: Cochrane Library; Web of Science; ProQuest; CINAHL; Arts and Humanities; Ebsocohost; Pubmed; PsycINFO. Other databases were also used including Google Scholar and to reduce the possibility of publication bias. The Grey literature was also searched ( 30 ) using the websites of specific organizations, e.g., NHS Evidence, Kings Fund, Health foundation, Nuffield Trust and NESTA and University of Florida Arts-in Medicine Repository.
Screening: inclusion and exclusion criteria
The inclusion and exclusion criteria were developed using PICOC and SPICE frameworks ( 25 ) to ensure selection of relevant studies in the search. Studies meeting the following criteria were included: Participants/Population (P): articles with study populations of any age, from all countries and from those consisting of patients/service users and healthcare practitioners; Intervention (I): (i) specified referral routes in order to meet the definition of ‘arts on prescription’; (ii) participants were referred to community arts activities or interventions delivered by artists or other facilitators (e.g., museum education officers); (iii) Interventions were group arts activities, but all arts disciplines were included (for example visual arts, literary arts and performing arts); Comparison (C): Studies with and without comparison groups were included; Outcome (O): Reporting measures of impact (use of a validated tool to measure mental health symptoms, wellbeing, mental health, physical health outcomes, social isolation and/or loneliness) and/or articles with qualitative accounts of patients/service users and healthcare practitioners’ experiences; Context (C): (i) Community based or primary care-based studies; (ii) publications from 1994 (this corresponds to the first reported Arts on Prescription programme in the literature); (iii) studies written in English; Evaluation (E): include empirical data (quantitative, qualitative, or mixed methods studies).
Studies meeting the following criteria were excluded: Participants (P): No exclusion criteria; Intervention (I): (i) reporting on the expressive arts therapies (art, music, drama, dance) delivered as a psychotherapeutic intervention; (ii) reporting on community arts programmes without a referral process; Comparison (C): No exclusion criteria; Outcomes (O): No exclusion criteria; Context (C): based in inpatient/hospital-based and residential care home settings; Evaluation (E): case reports; opinion pieces and editorials, review papers and essays.
The protocol for screening was developed in March and April 2023, piloting tools and methods for sharing and saving data (e.g., Zotero and Mendeley). Literature search results were downloaded and shared in an Excel file, with a record of the screening process kept for each database. All titles and abstracts for each database search were saved on a separate Excel sheet, and the screening outcome for each study was recorded (whether included or excluded and a category for the reason for exclusion). All articles were screened for inclusion and exclusion criteria by the authors (AJ, HB, NH, SK) between July and September 2023, screening both abstracts and titles, and, where required, full texts of articles. All full text articles were available to authors. All study titles and abstracts identified by the searches were screened independently for inclusion in the review by two researchers using the study inclusion criteria. For studies that met the inclusion criteria, full text articles were independently screened and assessed for eligibility by the same two researchers. A third reviewer resolved any discrepancies. The final selection of studies was assessed and approved by all authors.
Quality assessment (and risk of bias)
Quality assessment of the selected articles was then undertaken by the authors (AJ, HB, NH, SK) between October and December 2023. The Critical Appraisal Skills Programme (CASP) templates (Qualitative studies template, and relevant type of Quantitative study, e.g., RCT or Cohort study) were used for qualitative and quantitative studies, and the Mixed Methods Appraisal Tool (MMAT) for studies that applied a mixed methods approach ( 26 , 27 ). For example, cohort studies are rated on items such as clarity of research aims, risk of bias, consideration of confounding variables, accuracy of results and their interpretation. Qualitative studies are rated on items such as validity of research design, reflection on relationship with participants and rigorous analytical methods. No Randomized Controlled Trials (RCTs) were identified in the search (see Tables 1 – 3 ) and therefore a specific Risk of Bias tool was not appropriate to use. Additionally, the CASP tool for quantitative cohort studies which was used to assess the quality of the identified quantitative studies includes two questions relating to bias, which was therefore assessed as part of the overall quality assessment. Two researchers independently rated the quality of each included paper with a third and fourth researcher helping to resolve any discrepancies identified. AJ, HB and NH have authored some of the included studies so were not involved in the quality assessment of those articles. The final ratings of studies were assessed and approved by all authors in a meeting where each paper and its criteria were discussed, to check for parity across studies.
Table 1 . Data extraction from qualitative studies.
Table 2 . Data extraction from mixed methods studies.
Table 3 . Data extraction from quantitative studies.
Data extraction (selection and coding)
A data extraction table was designed specifically for this systematic review on the basis of guidance from the Centre for Reviews and Dissemination ( 25 ). It included: population, intervention, context, and outcomes. In addition, details relating to study design and data collection methods were deemed relevant and included in the table ( 63 ). The data was extracted using an Excel template under the following headings: authors, year of publication and country; referral process; study setting and participant characteristics; study design and data collection method; intervention component/activities (including the frequency of sessions); results (including the outcome measures, qualitative themes or statistical analysis) and quality assessment (see Tables 1 – 3 ). Data for each study was extracted by one of the authors (AJ, HB, NH, SK) between October and December 2023 and checked independently by an additional author before data synthesis.
Data synthesis and analysis
A narrative review of results was undertaken, using multiple strategies to triangulate qualitative and quantitative outcomes. The qualitative results were synthesized using a thematic analysis approach. Data were closely examined to identify common themes – topics, ideas and patterns of meaning that came up repeatedly across studies ( 56 ).
Data that met the following criteria were included in a meta-analysis: included sufficient data (e.g., means and SDs) at pre and post programme points; had not already been presented in another paper; measured a conceptually homogenous outcome using validated measures. The meta-analysis was conducted with a pre-calculated effect-size, random-effects model within SPSS. Data, including means, standard deviations and standard errors were extracted from individual studies, and mean change scores (SDs and SEs) were computed. Egger’s regression-based test, and a ‘trim and fill’ analysis were used to assess publication bias. Heterogeneity in mean change scores was assessed with I 2 and variance across studies predicted with a meta-regression of all process variables common to included studies. Quality ratings were not included as a predictor since there was no variance in these (all being rated ‘medium’), with the same risk of bias issues in all studies (pre-post designs with no comparison groups). The outcomes of non-included studies, and additional, secondary outcome measures and process analyses in included studies, were summarized narratively, focusing on notable trends in the research.
Studies identified
The PRISMA chart ( Figure 1 ) shows the outcome of our database and research register search which identified 7,805 citations. Following title and abstract screening, and full text review of the 29 remaining articles, with application of the inclusion/exclusion criteria, 25 articles remained. As detailed above we also searched relevant websites and research repositories and through this process identified 1,094 research reports and articles. Following title and abstract screening, de-duplication and eligibility review, there was 1 additional research study to include in the final sample ( Figure 1 ).
Figure 1 . PRISMA flow diagram.
Data extraction: a summary of included studies
There was heterogeneity across study designs and measures used to assess the impact of interventions, as well as in the settings and range of activities offered in Art on Prescription programmes.
Study designs and measures
Ten studies were qualitative ( 31 , 33 – 39 , 48 , 67 ) using focus group interviews, one-to-one interviews, open question survey data and follow-up questions (see Table 1 ). Seven used mixed methods ( 40 – 46 ) (see Table 2 ). Eight studies used quantitative methods ( 32 , 47 , 49 – 54 ) (see Table 3 ).
Three of the mixed methods studies ( 44 – 46 ) and seven of the quantitative studies ( 32 , 49 – 54 ) used an observational pre-post intervention design, while Bergman et al. ( 47 ) included a treatment-as-usual comparator group (but with pre-follow-up measurement points only). A range of validated measures were used in the quantitative and mixed methods studies including the WEMWBS ( 55 ), Generalized Anxiety Disorder-7 (GAD-7) ( 61 ), Patient Health Questionnaire-8 (PHQ-9) ( 62 ), Hospital Anxiety and Depression Scale (HADS) ( 64 ), Stress Crisis Inventory-93 ( 68 ), Resilience Scale ( 56 ), frailty ( 69 ), loneliness ( 58 , 57 ), and measures of immediate mood: Short Mood Scale ( 70 ) and the UCL Museum Wellbeing measure ( 71 ). Some studies also used unvalidated measures including patient satisfaction surveys developed specifically for the project ( 36 , 41 , 42 ). All quantitative studies reported solely on the impact of interventions on participants.
Of the qualitative studies, seven used semi-structured interviews ( 33 – 35 , 37 – 39 , 67 ), one used in-depth interviews ( 38 ), one combined interviews and focus groups ( 48 ), one used focus groups alone ( 31 ), and one study responses to a qualitative survey ( 36 ). The average number of interviews were 9.8 per study. The focus of the majority of the qualitative and mixed methods studies was on the views of patients or services users. However, three qualitative and one mixed methods study reported on the perceptions of the healthcare professionals, and/or artists who had referred participants to the Arts on Prescription programmes ( 34 , 39 , 42 , 48 ).
Some studies used findings from the same empirical data set, including accruing data sets from Artlift (Gloucestershire, UK) ( 32 , 36 , 43 , 49 , 53 , 54 ); and Arts on Prescription programmes in Sweden and Denmark (Kulturvitaminer, Aalborg Kommune, DK) ( 31 , 33 , 47 , 67 ).
Programme settings and delivery
Most of the Arts on Prescription programmes were based in the United Kingdom (UK), with three in Sweden ( 31 , 34 , 47 ) two in Denmark ( 33 , 67 ), one in Australia ( 44 ), and one in the United States (US) ( 42 ). The settings were mostly described as community settings, primary care or a GP surgery, although one study took place during the COVID-19 pandemic and was therefore delivered remotely and one at a school ( 52 ).
Most studies worked with adult populations, although one study was conducted in a school with adolescents aged 13–16 ( 50 ) and one paper included a pilot intervention with young children ( 42 ). Adult participants tended to be older, with mean ages, where reported, being 43–57 years, although one study worked exclusively with participants 65+ with a mean age of 78 ( 44 ). Participants were predominantly female, although two qualitative studies included 50 and 70% male participants ( 37 , 38 ). Where reported, participants mostly identified as White British (77–80%). Some programmes reported a greater proportion of participants from lower socioeconomic groups ( 44 ) and a high proportion of participants described themselves as out of work or unemployed ( 49 , 52 ).
Referral processes varied, and were made by GPs and healthcare professionals in primary health care [e.g., Crone et al. ( 49 )] including psychiatric care ( 47 ), mental health care professionals in primary (and secondary) care sectors [e.g., Redmond et al. ( 37 )], pharmacists, pastoral carers and nurses ( 44 ), professionals from the voluntary sector and Job Centre staff ( 38 , 67 ), school mental health and pastoral care staff ( 50 ), and some studies included self-referrals [e.g., Crone et al. ( 49 )]. The most common reasons for referral to Arts on Prescription programmes were anxiety, depression, poor wellbeing, stress, including that associated with chronic illness and pain, loneliness or major life changes and loss, in addition to bullying, and difficult family situations in the school programme ( 50 ).
The length of the programmes varied between 8 to 12 weeks, apart from one programme where participation was available for up to 6 months ( 35 ), one programme of 20 weeks ( 46 ) and one programme that appeared to be ongoing ( 37 ).
A wide range of different arts activities were reported, including visual arts (photography, painting, sculpting, collage, mark making, mixed media); crafts (textiles, pottery, felting, green crafts); music (listening); singing (choir); dance and movement; literature (playwright, creative writing, poetry) as well as nature hikes, city walks, gallery and museum visits, object handling, film, theatre, and drama.
Study quality
In terms of study quality, two were scored to be of high quality, six studies were graded as being of low quality, and the remainder were scored to be of medium quality. Quality ratings for individual studies are detailed in Tables 1 – 3 . All quantitative studies received a ‘medium’ rating, most commonly due to: an absence of comparator groups, a lack of longitudinal data, unavailability of data on attrition rates and their impact, selection biases (since outcome data is available only for those who completed programmes), and missing data (e.g., at post-programme or follow-up points). This reflects the potential bias inherent in these studies. Observational studies such as those included in this systematic review are more susceptible to bias than experimental studies such as RCTs, and this is considered in the discussion and acknowledged in the conclusions to this review. These issues also pertained to the quantitative components of mixed-methods studies. Here, more studies received a ‘low’ rating, due to: due to a lack of detail regarding the research process, and the analysis and data being presented in a superficial manner, use of unvalidated psychometric tools, and qualitative data being based on brief comments on evaluation questionnaires rather than in-depth focus groups or interviews. The qualitative research was generally of a higher quality, with two studies receiving a high rating, reflecting clear research aims, interpretation of results, rigor of analysis and reflexivity during the process. Common problems with the qualitative research, however, leading to medium ratings, regarded potential selection biases since participants were likely to be those who enjoyed and completed the programmes and a lack of consideration of the researcher’s role in the process (reflexivity).
The narrative review presents qualitative and quantitative findings separately in order to highlight the impact of Arts on Prescription on individual health and wellbeing from these distinct methods and research aims, without prioritizing one form of data over the other. The qualitative research focused on the subjective meaning of Arts on Prescription to participants, and the quantitative research on change in health and wellbeing over time, using psychometric measures of health and wellbeing from larger cohorts.
Qualitative findings
The qualitative outcomes were organized into three common themes among the Arts on Prescription studies. The themes are presented as impact on participants (social and psychological) and progression opportunities.
Social benefits
In terms of individual outcomes, the participants reported on common themes in terms of social benefits: social connectedness ( 31 ) and improved social skills and interaction ( 35 , 37 , 38 , 43 , 48 ), e.g., a participant specified; “It’s helped me interact with people more, this course has, and yeah, I’ve made lots of close friends so it’s really good” ( 33 , p. 577). Other social gains were experienced as the ability to foster the development of meaningful relationships with others ( 44 ), increased social confidence ( 36 ), sharing experiences and normalising emotions ( 42 ) as well as breaking and decreasing social isolation ( 41 , 42 ), as a participant stated: “[…] interacting with other people that also helps people in their recovery […]Or, even maintaining wellbeing, interacting with others […]” ( 41 , p. 280) and further building a sense of community ( 45 ).
However, some participants also reported finding the social setting difficult to manage (e.g., feeling disconnect from others and having social anxiety) ( 39 , 43 ) which perpetuates the discussion about how Arts on Prescription programmes should be delivered, and the negative sides of group interaction ( 10 , 72 ) and emphasises that one-size does not fit all when it comes to health promoting activities, where individual and cultural preferences should be considered ( 10 ). According to a participant: “Other participants can make you feel uncomfortable” ( 39 , p. 10). Others reported participation limitations due to physical barriers, e.g., tremors from medication or being a slow thinker ( 39 ).
Psychological benefits
Participants reported various psychological benefits including increased self-confidence ( 37 , 41 , 42 , 67 ), improved self-esteem ( 45 ) and a sense of achievement ( 41 , 44 ). A participant specified:” It’s the best thing I’ve done. It’s given me confidence […] since I’ve started art. I have started volunteering again […]” ( 33 , p. 577). Participants also experienced engagement and pleasure ( 33 ) and described arts activities as positive distractions, enabling absorption and forgetting worries and concerns ( 43 ). In terms of self-efficacy ( 33 , 37 , 42 ) participants reported a move from self-critical to self-caring as a participant expressed” […]I’ve a better sense of myself and self-esteem and do things that are good for me […] ( 67 ). Others reported gaining and increasing motivation ( 37 , 43 ) empowerment ( 44 ) and control in life ( 41 ).
However, one study also found that positive wellbeing outcomes (relaxation and distraction) varied according to the nature of the interactions between individuals in the Arts on Prescription group ( 43 ) – which indicates that group dynamics and group facilitation are important for psychological benefits to occur ( 10 ).
Progression opportunities
Another theme that occurred across different studies related to participants’ progression. Progression outcomes on mental and spiritual levels were reported as an ability to determine a new future ( 38 ); expanding worlds, as a participant expressed: “It has given me a desire to experience more. It is as if the world has become bigger, I think ” ( 31 ), p. 5; accessing new worlds, assuming and sustaining new identities ( 37 ); health promoting changes ( 31 ) and positive changes in life ( 67 ). On a more practical level, progression meant joining new activities ( 41 ), and a shift to gain a sense of direction ( 44 ) and returning to normality ( 35 ). As stated by a participant: “[…] feeling normal…it’s not feeling tired, achy, sad […] ” ( 48 ), p. 70. Another participant further stated: “ It’s moved me on ” ( 67 ), p. 11.
However, some participants also reported feeling anxious about the end of the programme and losing the support from the group ( 43 ). The negative consequence of a lack of new opportunities and further pathways for Arts on Prescription participants was considered in various studies ( 10 , 36 , 43 ) Authors of an Arts on Prescription study identified that “ participants also perceived the inevitability of the course ending as a cause for concern. They felt anxious about the prospect of losing the support structure that they relied upon and lacked the confidence to maintain their health and wellbeing on their own ” ( 39 , p. 13).
Summary of qualitative outcomes
The qualitative findings illustrate that across the studies participants experienced positive social and psychological results and progression outcomes on mental and spiritual levels as well as on practical levels. However, the participants also reported feeling anxious about the ending of the programme and, for some, the negative impact of group dynamics.
Quantitative outcomes
All 15 quantitative and mixed-method studies reported improvements in outcomes across Arts on Prescription programmes ( Tables 2 , 3 ). This included improvements in wellbeing in twelve studies ( 32 , 40 , 41 , 43 , 44 , 46 , 49 – 54 ) reductions in symptoms of anxiety and depression in two studies ( 47 , 54 ), and improvements in a range of additional outcomes (including resilience, creativity, loneliness, mood; but not frailty or satisfaction with relationships) ( 44 , 45 , 50 , 52 ). Some studies focused on process rather than outcome factors, examining data sets for predictors of wellbeing change, including demographics, wellbeing at baseline, and experience during art workshops as predictors ( 43 , 46 , 49 , 51 – 53 ). A meta-analysis of primary outcomes and a narrative review of additional outcomes (secondary outcome measures, longitudinal data and process factors) is presented below.
Meta-analysis: improvements in mental wellbeing
Only seven out of 15 quantitative/mixed methods studies met the inclusion criteria for the meta-analysis, the most common reason being due to duplicated data ( 32 , 40 , 43 , 53 ), followed by insufficient data ( 41 , 42 , 47 ), including having no data immediately post-intervention; and one heterogeneous outcome (momentary mood rather than long-term wellbeing) ( 45 ). All seven included studies assessed subjective wellbeing, using the WEMWBS as an outcome measure. The WEMWBS measures broad aspects of mental wellbeing, including cognitive, affective and social experiences (e.g., being able to concentrate, experiencing moments of joy and feeling connected to others) ( 55 ). Mean WEMWBS scores were typically low at the outset (ranging from 36 to 39, indicative of ‘low wellbeing’ in the scale’s normative data (the lowest 15% of scores being below 42)) [apart from in Poulos et al., ( 44 )]. Most studies reported a mean WEMWBS above 42 at the end of the art programme (means ranging from 41 to 57). Mean WEMWBS scores rose by 4 to 8 units, all of which are above that thought to indicate a minimally important level of change [a change of 3 units or more ( 73 )].
For the meta-analysis WEMWBS data (mean scores pre and post Arts on Prescription programmes, standard deviations and standard errors) were extracted from individual studies, and mean change scores (SDs and SEs) were computed. Mean change (between pre and post WEMWBS scores) was used as the effect size (for ease of interpretation, since all studies used the same outcome measure and did not require standardization). These effect sizes are plotted in Figure 2 . The Egger’s regression-based test, and no imputation being needed in a ‘trim and fill’ analysis, indicated that publication bias was not a problem in the sample ( 70 ). The overall mean wellbeing change was 5.82 (SE = 0.471), which was statistically significant ( Z = 12.357, p <. 001, 95% CI 4.90–6.748). Even the low estimate of the 95% confidence interval indicates a mean difference of 4.9, which is greater than the minimally important level of change (of 3) ( 57 ), reflecting the significant improvement in wellbeing in all individual studies.
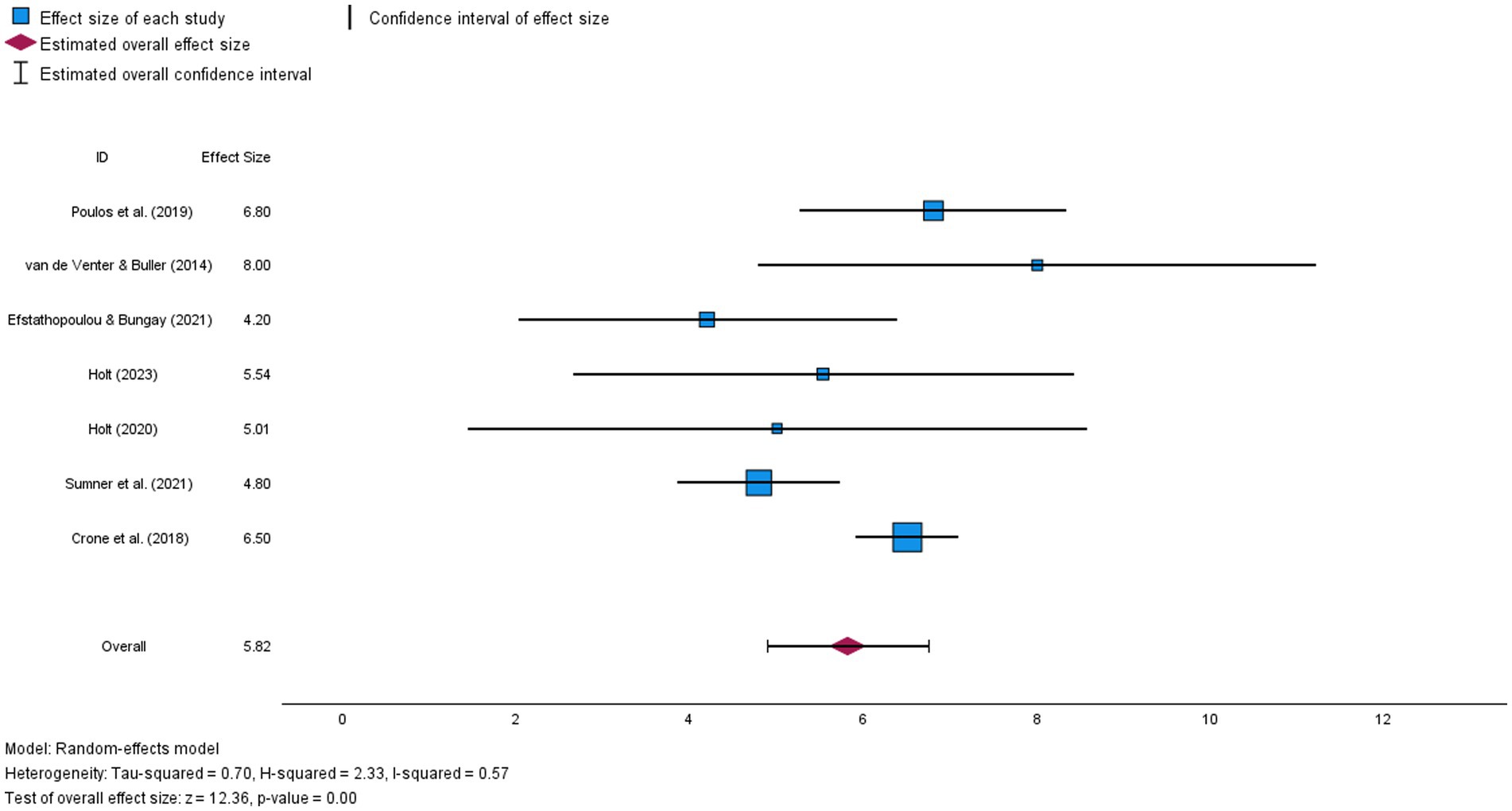
Figure 2 . Forest plot illustrating mean wellbeing change (and confidence interval) for each study in the meta-analysis and estimated overall effect size.
Despite this, there was significant heterogeneity in mean change scores ( Q = 14.581, df = 6, p = 0.024), with an I 2 of 57% (a moderate effect size, indicating that 57% of the variance in mean change scores between studies could not be explained by sampling error). Hence, variation in the efficacy of programmes could be explained by other factors, such as participant characteristics (age, reasons for referral, baseline wellbeing), intervention settings (e.g., schools, remote delivery, GP surgeries, community hubs) or intervention processes (e.g., group sizes, art activities, length of programmes, participant rapport or engagement).
A meta-regression with process variables available for all studies (baseline wellbeing, programme length, mean age and % female) was therefore conducted to test whether these explained variance in mean wellbeing change across studies. With their inclusion the unexplained residual heterogeneity was no longer statistically significant ( Q = 1.839, df = 2, p = 0.339). No individual predictors were independently statistically significant (possibly due to low statistical power with only seven studies). Nevertheless, as indicated in Table 4 , studies with larger mean changes in wellbeing were more likely to have: longer programmes, older participants, participants with lower mean WEMWBS scores at baseline, and a lower % of females in groups (%s ranging from 67 to 92); the strongest effect size being for programme length.
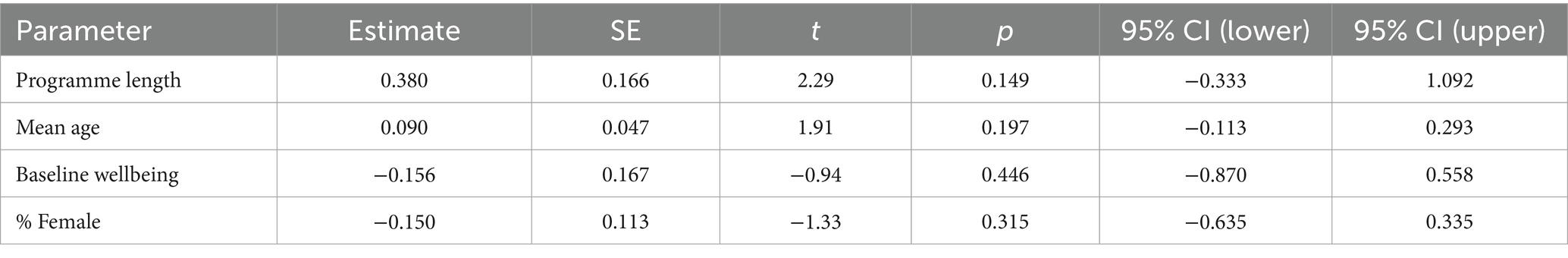
Table 4 . Parameter estimates and statistical significance for meta-regression, with the effect estimate of mean wellbeing change.
Impact on secondary and heterogeneous outcomes
The consistent increase in wellbeing scores reported across Arts on Prescription programmes was supported by further studies showing statistically significant improvements in clinical and social outcomes. These included symptoms of anxiety and depression ( 47 , 54 ), mood ( 45 ) and feelings of loneliness (but not satisfaction with relationships) in a remotely delivered Arts on Prescription programme ( 52 ). In the school Arts on Prescription intervention ( 50 ) resilience scores significantly increased, indicating that pupils felt better able to cope with adversity (regulating reactions and persevering despite setbacks) ( 56 ). In Poulos et al.’s ( 44 ) study with older adults, self-perceived creativity and engagement with creative activities significantly increased following the arts programme, however, frailty including self-reported exhaustion, slow walking speed and grip strength ( 69 ) did not.
In the only study with a comparator condition, Bergman et al. ( 47 ) examined the impact of Arts on Prescription for patients on sick leave due to common mental disorders and/or musculoskeletal pain. Patients completed measures of stress (SCI-93) and anxiety and depression (HADS) at baseline and at follow-up (either 6 or 12 months after the intervention). Participants were referred to Arts on Prescription programmes in the usual way, and control participants were receiving treatment as usual, and were invited through a stratified selection process of people meeting the study’s inclusion criteria. Both groups reported a significant reduction in both stress and anxiety and depression across time points. However, the Arts on Prescription group reported greater reductions, which reached statistical significance for anxiety and depression. This supports the use of Arts on Prescription for the reduction of anxiety and depression, in line with Sumner et al. ( 54 ). However, since clinical outcomes were not taken at the end of the ten-week-long intervention, it is not clear what the immediate impact of participation was on symptoms.
Longitudinal impact
As discussed above, Bergman et al. ( 47 ) reported that anxiety and depression was lower than at baseline for participants at a follow-up point (either 6-or 12-months post-intervention). One further study included a follow-up data point, at 3 months ( 50 ). There was no significant increase in either wellbeing or resilience compared to baseline at this stage. However, this outcome was difficult to interpret due to large amounts of missing data at follow-up.
While there is little longitudinal data available on Arts on Prescription, two additional studies examined the impact of re-referrals ( 51 , 54 ). For re-referred participants there were significant improvements in wellbeing, anxiety and depression across programmes, and a significantly higher level of wellbeing than at the end of one referral period alone ( 51 ), suggesting accrued benefits of longer attendance. However, there was a rebound to lower levels of wellbeing between referral cycles in both studies. It is important to note that these participants have been re-referred and thus are thought to require further support. Further work is required to identify whether this rebound occurs for all participants and to investigate the longitudinal impact of Arts on Prescription. The long-term impact of engagement is difficult to discern from these limited and mixed outcomes.
Process factors
Several quantitative studies included analysis of process factors, seeking to identify good practice and ‘active ingredients’ ( 43 , 46 , 49 , 51 – 53 ). It is important to note that while average improvements in wellbeing across programmes have been consistently reported, not all individuals report improvements in wellbeing ( 46 , 51 , 52 ). Several studies have examined whether patient characteristics or process variables predict the extent of wellbeing change. Statistically significant predictors of wellbeing change in these studies: attendance ( 53 ) an 8 versus 10-week-long programme ( 49 ); lower baseline wellbeing scores ( 49 ); a reduction in anxiety during the art workshops; being able to get absorbed in the art making (enter the ‘flow state); feeling less lonely during art workshops ( 51 , 52 ); and ethnicity (with a greater increase for participants identifying as BME) ( 46 ). Neither the presence of multi-morbidities (multiple medical complaints) nor additional demographic data have predicted the extent of wellbeing change ( 53 ), suggesting that Arts on Prescription is widely beneficial across referral types. Overall, these outcomes suggest that features of programmes (e.g., length), participants (e.g., wellbeing) and engagement with workshops (e.g., attendance and getting absorbed in activities) affect the extent to which wellbeing is improved.
Summary of quantitative outcomes
Overall, these quantitative outcomes support the use of Arts on Prescription for improving mental wellbeing, with an overall mean wellbeing change of 5.82, and with some support for reduction of anxiety and depression ( 47 , 54 ). Strengths of the research include several studies using the same measures, enabling direct comparison, high ecological validity (being based in primary care and community settings), and useful research on process variables, which inform best practice. However, limitations with the studies are varied and include an absence of comparator groups, a lack of longitudinal data, and unavailability of data on attrition rates and their impact in several studies. These quality issues will be further explored in the discussion.
To the authors’ knowledge this is the first systematic review reporting the wellbeing impact of participating in Arts on Prescription. Evidence from across the 25 studies included in the review indicates that Arts on Prescription has both a meaningful personal impact and statistically significant impact on subjective wellbeing. The narrative review of qualitative studies supported the view that participants found Art on Prescription helped to improve social and psychological wellbeing, and that this ‘rippled out’ to affect lives beyond the programmes. The meta-analysis supported this with a significant increase in wellbeing across included studies, while the narrative review of secondary outcomes suggested a role for reduction in anxiety and depression, and suggested useful process factors that increase ‘wellbeing change’ across programmes. These findings support other reviews on the benefits of engaging with the arts for mental health in other settings and suggest that Arts on Prescription is an appropriate intervention for improving psychosocial wellbeing ( 74 – 76 ). However, there are numerous caveats with the evidence base and challenges with delivery and development of best practice, which will be considered below.
Impact of arts on prescription on health and wellbeing
Although the pre-post quantitative data show the effectiveness of Arts on Prescription on well-being, the qualitative data from across the mixed methods and the qualitative studies add an indication as to why this may be the case. Across these studies there were reports of psychological and social benefits. Participants reported enjoyment and pleasure which are linked to the hedonistic elements of wellbeing ( 77 ). Furthermore, there were examples of a sense of purpose, meaningful engagement, absorption in art activities, and self-development through increased self-confidence and self-esteem, which contribute to eudaimonic wellbeing ( 78 , 79 ). In addition, participants were able to develop relationships, which may also reduce loneliness, and contribute to a sense of relatedness, fundamental to wellbeing ( 80 ). Quantitative process research added to this qualitative research, suggesting that multiple, independent mechanisms lead to wellbeing change across Arts on Prescription programmes: feeling less anxious in art workshops; getting into an absorbed attentional state; and feeling connected to others ( 51 , 52 ). These outcomes suggest that by engaging in Arts on Prescription participants have the opportunity to develop psychosocial components that are central to numerous models of wellbeing, for example, the five components of the PERMA model, where wellbeing is constituted by: (1) experiencing positive emotional states in everyday life; (2) getting deeply involved and absorbed in meaningful activities in everyday life; (3) positive relationships and interactions with others; (4) a sense of purpose and meaning in life; and (5) a sense of accomplishment, of self-efficacy, working toward and reaching goals in everyday life ( 79 ).
The qualitative work suggests that participants felt that this pathway to wellbeing was enabled by the creation of a ‘safe space’ by the arts facilitator [e.g., Stickley and Hui ( 38 )], where it was safe to ‘play’ and create, and through social bonding ( 72 ). Hughes et al. ( 43 ) proposed a process of change model, where social bonding enables subsequent psychological benefits, since feeling socially safe, allows relaxation and opportunities to go into a state of ‘flow’ while making art, unlocking mechanisms for eudaimonic wellbeing ( 78 ). As such, the review suggests that Arts on Prescription improves wellbeing, as part of a ‘social cure approach’ ( 72 , 81 ), but that this is only part of the picture, with additional psychological mechanisms also being important.
Beyond the immediate impact of the programmes Arts on Prescription was perceived in qualitative research to act as a catalyst into other activities ( 67 ) that continued to develop wellbeing (although opportunities for other programmes were described as limited). This raises the issue of how to best support individuals at the end of programmes, and the potential negative consequences for wellbeing that could arise for some at the end of the group, if other opportunities, such as ‘move on’ community art groups are not available ( 10 , 58 ). It also reminds us that there is very little research on Arts on Prescription from a longitudinal perspective, nor of the factors that are required to maintain wellbeing after the end of programmes.
Barriers and challenges
Whilst most of the studies focused on reporting the benefits and positive impact of the Arts on Prescription programmes there were some that also reported barriers to participation. These barriers for individual participants included difficulties with access, such barriers could be physical, socio-economic and/or psychological. There were also barriers to recruitment identified which were linked to health care professionals’ awareness and perceptions about the service, but also potential systemic barriers due to funding and commissioning of services ( 42 , 44 ).
Physical barriers to attending Arts on Prescription included transportation for participants, this was an issue for some specific groups such as children needing arrangements to be made with parents or guardians, and for those with mobility issues for example Parkinson’s Disease, parking was also reported to be an issue at some venues ( 42 ). Poulous et al. ( 44 ) also questioned whether physical frailty and limited mobility were potential barriers to access for the older people living in the community (the target group for their Arts on Prescription programme). There were similar issues cited by participants in Hughes et al.’s ( 43 ) study who reported physical limitations as barriers, likewise Crone et al. ( 49 ) reported that patients with multimorbidities were less likely to attend. Mental ill health factors were also potential risks to being able to attend the sessions.
Both the qualitative studies and the quantitative process-research identified potential barriers to participation. Individuals with lower levels of wellbeing at the start of programmes were found to have more difficulties attending and engaging, as were those with more reasons for referral (multimorbidities), and both higher and lower levels of deprivation ( 49 ). Levels of wellbeing, anxiety and depression were very low at baseline for some participants, hence Sumner et al. ( 53 ) raised concerns about screening for participation, given that people with high levels of depression/anxiety may struggle to engage and may be ‘set up for failure’ ( 49 , 54 ).
Low participant engagement has also been identified as an issue in some Social Prescribing projects. For example, Pescheny et al. ( 7 ), identified that patients’ lack of interest and scepticism about the potential benefits of Social Prescribing, preference for a medical solution, transport issues and concern with stigma due to links with mental health services explained low engagement in two of the evaluations included in their systematic review. Fear of stigmatization may also be associated with interventions that explicitly target loneliness and can unintentionally create barriers to accessing support. However, it has been suggested ( 82 ) that group activities that connect people, but are not explicitly targeted at reducing loneliness, could be a solution to this. Arts on Prescription programmes foster social connections and may therefore be a suitable intervention, but as Bungay et al. ( 10 ) highlight, not all people enjoy sharing group practices and the group may not be perceived to be a positive safe space for some and may reinforce feelings of social isolation.
Most of the Arts on Prescription programmes featured in the current review were held face to face, but during the COVID-19 pandemic some moved to remote delivery formats, and this could be a good alternative at ‘normal’ times for those experiencing physical barriers to access due to transport issues, or psychological barriers due to reluctance to leave the house. Research by Holt ( 52 ) looked at experiences of remote Arts on Prescription workshops, the pre-post intervention design identified that global wellbeing improved and there was also a reduction in loneliness, but it needs to be acknowledged that lack of access to digital resources may be an issue for some and that a ‘going somewhere’ and ‘physically meeting’ others might be an important element of the Arts on Prescription programmes.
Disadvantaged communities may experience barriers to accessing online resources, indeed, Golden et al. ( 42 ) found that where online activities were offered this was a barrier for those lacking digital access. They also reported that where English is the primary language used in cultural venues it could be problematic for those for whom English is a second language and for those with literacy issues within diverse populations.
Sumner et al. ( 54 ) found that deprivation was associated with attendance, with those from the median deprivation quintile being more likely to attend. For some ‘arts’ and cultural spaces may be considered elitist and as a result people may feel excluded and that they do not belong in such places or the ‘arts’ aren’t for them and feel intimidated in cultural spaces. As stated above, exclusion or sense of belonging may also be due to the group dynamics with not everyone having a positive experience of being in a group with some feeling that they do not belong in the group or feel excluded ( 72 ). Similarly, not all people see themselves as ‘creatives’ ( 44 ) and this may present a further barrier to attendance. People with chronic mental health problems may experience persistent difficulties with ‘going out’ and re-engaging with everyday life prior to attending the programme ( 35 ). Crone et al., ( 49 ) also found that those with lower levels of wellbeing at baseline were less likely to attend the programme and called for further research to explore the reasons behind this, although this may be explained by the difficulties faced by some in ‘re-engaging with everyday life’. This gives some indication as to why not all those referred to Arts on Prescription programmes attend, as they need to overcome those hurdles to be able to engage with the group.
Where gender was reported, the Arts on Prescription programmes appear from the studies in this review to be dominated by women with relatively few men taking up the opportunity [other than: Stickley and Hui ( 38 ); Stickley and Eades ( 37 )]. For example, in Bergman et al. ( 31 ) all the participants were women, in Poulous et al. ( 44 ) 74% were women, and in Crone et al. ( 49 ) 79% were women. Women are more likely to join community-based social groups than men ( 83 ) and this may explain the preponderance of women in most of the studies. However, no detail is provided in any of the studies as to whom is offered Arts on Prescription, so it is not possible to determine if there is an inherent bias due to more women than men being offered the opportunity to attend the programmes.
In terms of attending the sessions Crone et al. ( 32 ) suggested that the reason why older participants engaged in the programmes in their study was that older people were more likely to attend GP surgeries – and explain the older participants in their sample. However, this was not reflected across all the studies reviewed. The demographic characteristics of the populations in the reviewed studies suggests the need for further research to look at who is referred to Arts on Prescription and who attends and engages once referred.
The duration programmes, in terms of the number of weeks across which sessions ran, was reported as a factor which may impact on people attending ( 49 ). In their research it was found that when the duration of the course was cut participation and the wellbeing scores increased. However, in Jensen and Bungay ( 34 ) health professionals reflected that recovery from mental health problems took time and suggested that a ten-week programme could only be a starting point to get people motivated to do other things. Further, in this review, it was noted that higher levels of wellbeing were reported at the end of programmes that ran for a longer period of time, and for participants who were re-referred to a second programme ( 46 , 51 , 54 ). Another important finding of Crone et al. ( 49 ) was that greater choice of locality, art type and activity lead to higher levels of engagement with the programme. Therefore, careful consideration of the delivery and structure of a programme, locations, and what is offered may support recruitment and engagement.
In terms of systemic barriers to Arts on Prescription programmes, one of the earliest reports on an evaluation of an AoP programme (‘Time Being’ on the Isle of Wight) by Eades and Ager ( 41 ) outlined the difficulties in integrating arts as healthcare. What is interesting in this article is the discussion section that focuses on the political and institutional challenges faced when trying to establish and maintain a programme. This included structural changes to the NHS and whilst ‘Time Being’ was found to have positive impacts on participants over the years that it ran, the NHS and Primary Care Trusts became focused on economic impacts, and for them to commission new activity, including arts as health programmes, required the demonstration of health gains, cost benefits, and service improvements. Those referring to an existing Arts on Prescription programme referred to the need to evidence the outcomes ( 39 ) and expressed hope that Arts on Prescription became regarded as cost effective, also recognising the need for the service to be commissioned by General Practitioners for it to continue. This still has resonance today as there is a continuing issue for organizations seeking funding to establish Arts on Prescription or similar programmes of activity as part of the Social prescribing offer more widely.
Eades and Ager ( 41 ) also reported that at the beginning of ‘Time Being’ it was difficult to engage medical professionals fully and gain recognition for the potential benefits of Arts on Prescription. Likewise in Australia, Poulous et al. ( 44 ), found it necessary to raise healthcare practitioners’ awareness of an Arts on Prescription programme to promote recruitment. This took considerable effort, both to raise awareness in the community and to educate health care practitioners about its potential uses and benefits. For the implementation of social prescribing interventions Pescheny et al. ( 7 ) suggested a phased approach to the rollout of programmes, because it provides time for the development of effective partnerships between General Practitioner surgeries and third sector organizations. Participants in Jensen and Bungay’s ( 34 ) study, however, suggested that because of time pressures and stressors in primary care, practitioners may forget about alternative interventions to alleviate mild to moderate depression. Furthermore, it was also suggested that referral to Arts on Prescription required a shift away from the dominant medical model of health to a more holistic social model of care.
Critical issues with the evidence base
In addition to knowing very little about how artist facilitators run the various art programmes reviewed here, there is very little knowledge about how the arts programmes are designed, for example, what specific activities were included, and why, whether the skill required to complete art activities was scaffolded across programmes, etc. If we want to understand more about the various arts’ impact on the individual and to design programmes with maximum benefit, it is imperative to know whether specific arts activities may help in different ways, need to be approached and introduced in certain ways, and whether there are different outcomes by offering, e.g., participants 10 weeks focusing just on visual arts [e.g., Crone et al., ( 32 )] versus a programme that includes various art activities (literature, theatre, dance, music, etc.) at different locations ( 67 ). Clarification of these issues could draw and build upon experimental work on the arts and health, showing that the arts can be used in different ways for different health outcomes, where, for example, greater mood improvement and engagement is achieved when art activities match skill levels ( 84 , 85 ).
While the current review supports the use of Arts on Prescription for improving psychosocial wellbeing, a number of caveats with the evidence base must be considered. Firstly, the quality rating of most studies was medium, and none of the quantitative studies were rated as ‘high’. This reflects the challenges with meeting the requirements of RCTs (especially when Arts on Prescription is a ‘personalized prescription’), with selection biases in those who participate in the completion of questionnaires and interviews, with unreported attrition rates in many studies (potentially leading to a biased sample), and with potential reporting biases and demand characteristics (where participants may complete end of programme questionnaires with an expectation to feel better, or wish to demonstrate this to artist facilitators). While Egger’s regression-based test indicated there was not a problem with publication bias in the studies included in the meta-analysis, it is still possible that studies with positive changes in wellbeing were more likely to be reported, and reporting and sharing of all outcomes should be encouraged, and a large pre-registered trial, across different Arts on Prescription programmes would be useful. Further research with comparator groups (e.g., treatment as usual), or wait-list groups, would be useful to help control for contextual variables [following Bergman et al. ( 47 )], as would research with active comparator groups (e.g., low intensity group therapy), to help identify the specific benefits of Arts on Prescription. However, other methods could help to improve the evidence base, for example methods and ecological momentary assessment designs, where individuals can act as their own controls in ‘non-treatment’ periods and more data on longitudinal and process factors can also be collected ( 4 ).
Our review points to a number of gaps in the current evidence base. For example, it is not clear who Arts on Prescription works best for, and whether Arts on Prescription may be useful for other reasons than to improve psychosocial wellbeing, for example, to help people to manage chronic conditions. More research is required using specific outcome measures such as anxiety and depression ( 54 ) and loneliness ( 52 ), as well as with both specific and more diverse populations (e.g., children and young people, male groups, people with chronic health conditions). Further, there has been a reliance on the use of the WEMWBS, partly due to its ease of administration, but consideration of more complex, dimensional models of wellbeing, as discussed above (e.g., the PERMA model) could increase understanding of how, and in what way, Arts on Prescription impacts wellbeing ( 76 , 79 ).
We acknowledge that other research papers are relevant in the category of SP programmes that offer cultural activities and which add to our understanding of the cultural benefits of the arts [see for example Todd et al. ( 86 ) and Thomson et al. ( 87 )] for Museums on Prescription, Helitzer et al. Singing on Prescription ( 88 ). However, the studies did not meet the inclusion criteria for this review [e.g., being a review paper ( 88 )], or being outside the scope of search terms due to focusing on ‘non art’ based museum activities (such as object handling or reminiscence activities based around Museum exhibits), due to referral processes not being specified or due to not being group activities ( 86 , 87 ).
Strengths and limitations
We used clearly defined aims and followed PRISMA reporting guidelines for this systematic review. However, we did not examine trial websites. As we are familiar with the area of Arts on Prescription, we have increased confidence that the available evidence has been identified. We also applied standardized tools for the appraisal process (CASP, MMAT). The synthesis of the studies has allowed for identification of qualitative themes and a meta-analysis and narrative review of quantitative outcomes.
However, it is possible that some grey literature that has not been published in formal ways (i.e., books and journals), could have been missed, although we also searched the websites of organizations. A range of keywords were used for the searches; however, studies might have been missed due to incorrect categorising or indexing in the databases. The review was limited to English language publication and other relevant studies may have been published in other languages.
Conclusion and future perspectives
The current review supports the use of Arts on Prescription for the improvement of psychosocial wellbeing. Quantitative data consistently reported improvements in health and wellbeing, and qualitative studies reported that participants found Arts on Prescription meaningful, helping to improve their psychological and social wellbeing. While this suggests that Arts on Prescription is a useful intervention in primary care settings, limitations with the evidence base, and barriers to engagement reported on in the review must also be considered. These include a reliance on observational quantitative studies and selection biases in qualitative research. Further, Arts on Prescription participants have tended to have a limited demographic (being mostly female, older and white), and reasons for this lack of diversity need to be investigated and remedied. Numerous barriers to engagement have been suggested, including physical, psychological, and social barriers, which have implications for practice. More work seeking to understand the nature of art activities that are offered and their impact, and to develop good practice and training for Arts on Prescription facilitators is required. Further research is required to include comparator groups, improve understanding of longitudinal impact and mechanisms by which Arts on Prescription improves wellbeing. Synthesizing the best available evidence on Arts on Prescription programmes, we hope this review is considered useful both in terms of practice and policy making.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
AJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. NH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SH: Data curation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. HB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bungay, H, and Clift, S. Arts on prescription: a review of practice in the UK. Perspect Public Health . (2010) 130:277–81. doi: 10.1177/1757913910384050
PubMed Abstract | Crossref Full Text | Google Scholar
2. Chatterjee, HJ, Camic, PM, Lockyer, B, and Thomson, LJM. Non-clinical community interventions: a systematised review of social prescribing schemes. Arts Health . (2018) 10:97–123. doi: 10.1080/17533015.2017.1334002
Crossref Full Text | Google Scholar
3. Jensen, A, Stickley, T, Torrissen, W, and Stigmar, K. Arts on prescription in Scandinavia: a review of current practice and future possibilities. Perspect Public Health . (2017) 137:268–74. doi: 10.1177/1757913916676853
4. Holt, N, Matthews, J, and Elliot, C. Art on prescription: practice and evidence In: P Crawford and P Kadetz, editors. Palgrave encyclopedia of the health humanities . Cham: Springer International Publishing (2024)
Google Scholar
5. Costa, A, Sousa, CJ, Seabra, PRC, Virgolino, A, Santos, O, Lopes, J, et al. Effectiveness of social prescribing programs in the primary health-care context: a systematic literature review. Sustain For . (2021) 13:2731. doi: 10.3390/su13052731
6. Muhl, C, Wadge, S, and Hussein, T. Social prescribing and students: a scoping review protocol. PLoS One . (2023) 18:e0289981–1. doi: 10.1371/journal.pone.0289981
7. Pescheny, JV, Randhawa, G, and Pappas, Y. The impact of social prescribing services on service users: a systematic review of the evidence. Eur J Pub Health . (2020) 30:664–73. doi: 10.1093/eurpub/ckz078
8. Muhl, C, Mulligan, K, Bayoumi, I, Ashcroft, R, and Godfrey, C. Establishing internationally accepted conceptual and operational definitions of social prescribing through expert consensus: a Delphi study. BMJ Open . (2023) 13:e070184. doi: 10.1136/bmjopen-2022-070184
9. Pescheny, JV, Pappas, Y, and Randhawa, G. Facilitators and barriers of implementing and delivering social prescribing services: a systematic review. BMC Health Serv Res . (2018) 18:86. doi: 10.1186/s12913-018-2893-4
10. Bungay, H, Jensen, A, and Holt, N. Critical perspectives on arts on prescription. Perspect Public Health . (2023) 12:175791392311707. doi: 10.1177/17579139231170776
11. Dingle, GA, and Sharman, LS. Social prescribing: a review of the literature. In: Menzies RE, Dingle GA, editors. Menzies RG, Existential concerns and cognitive-behavioral procedures . Cham: Springer (2022).
12. Walker, K, Griffiths, C, and Jiang, H. Understanding the underlying mechanisms of action for successful implementation of social prescribing. Open J Prev Med . (2023) 13:41–56. doi: 10.4236/ojpm.2023.132004
13. Woodall, J, Trigwell, J, Bunyan, AM, Raine, G, Eaton, V, Davis, J, et al. Understanding the effectiveness and mechanisms of a social prescribing service: a mixed method analysis. BMC Health Serv Res . (2018) 18:604. doi: 10.1186/s12913-018-3437-7
14. Vidovic, D, Reinhardt, GY, and Hammerton, C. Can social prescribing Foster individual and community well-being? A systematic review of the evidence. Int J Environ Res Public Health . (2021) 18:5276. doi: 10.3390/ijerph18105276
15. Napierala, H, Krüger, K, Kuschick, D, Heintze, C, Herrmann, WJ, and Holzinger, F. Social prescribing: systematic review of the effectiveness of psychosocial community referral interventions in primary care. Int J Integr Care . (2022) 22:11. doi: 10.5334/ijic.6472
16. National Health Service. Social prescribing. (2022). Available at: https://www.england.nhs.uk/personalisedcare/social-prescribing/
17. Sanderson, J, Kay, N, and Watts, R. Universal personalised care . London: Implementing the comprehensive model (2019).
18. Department for Culture, Media, and Sport. A connected society: A strategy for tackling loneliness . London: (2018).
19. Scottish Government. A connected Scotland: Our strategy for tackling social isolation and loneliness and building stronger social connections . Edinburgh: Scottish Government (2018).
20. Welsh Government. Connected communities: A strategy for tackling loneliness and social isolation and building stronger social connections . Cardiff: Welch Government (2020).
21. The Royal Australian College of General Practitioners (RACGP). Social prescribing roundtable, November 2019: Report . (2020). Available at: https://www.racgp.org.au/FSDEDEV/media/documents/RACGP/Advocacy/Social-prescribing-report-and-recommendation.pdf
22. Jensen, A. Kultur og sundhed. Et mangfoldigt felt. Forskning, teori og praksis . Aarhus: KLIM (2022).
23. PROSPERO. The impact of arts on prescription on individual health and wellbeing: A systematic review . (2023). Available at: https://www.crd.york.ac.uk/prospero/export_details_pdf.php
24. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. Statement: an updated guideline for reporting systematic reviews. BMJ . (2020) 2021:n71–1. doi: 10.1136/bmj.n71
25. Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care . UK: Centre for Reviews and Dissemination. York (2008).
26. Critical Appraisal Skills Programme. Available at: https://casp-uk.net/casp-tools-checklists/ . (2023).
27. Hong, QN, Pluye, P, Fàbregues, S, and Bartlett, G. Boardman F . Mixed Methods Appraisal Tool (MMAT): Cargo M (2018). Available at: https://www.nccmt.ca/knowledge-repositories/search/232
28. Higgins, J, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, M, et al. Cochrane handbook for systematic reviews of interventions . 2nd ed. Chichester (UK): John Wiley & Sons (2019).
29. Sonke, J, Manhas, N, Belden, C, Morgan-Daniel, J, Akram, S, Marjani, S, et al. Social prescribing outcomes: a mapping review of the evidence from 13 countries to identify key common outcomes. Front Med . (2023) 10. doi: 10.3389/fmed.2023.1266429
30. Rosenthal, R. Meta-analytic procedures for social research. Applied social research methods series . Beverly Hills, CA, USA: Sage Publications (1984).
31. Bergman, P, Jansson, I, and Bülow, PH. ‘No one forced anybody to do anything – and yet everybody painted’: experiences of arts on referral, a focus group study. Nord. J. Arts Culture Health . (2021) 3:9–20. doi: 10.18261/issn.2535-7913-2021-01-02-02
32. Crone, DM, O’Connell, EE, Tyson, PJ, Clark-Stone, F, Opher, S, and James, DVB. ‘Art lift’ intervention to improve mental well-being: an observational study from UK general practice. Int J Ment Health Nurs . (2013) 22:279–86. doi: 10.1111/j.1447-0349.2012.00862.x
33. Jensen, A, and Torrissen, W. Aesthetic engagement as health and wellbeing promotion. J Public Ment Health . (2019) 18:240–7. doi: 10.1108/JPMH-11-2018-0080
34. Jensen, A, and Bungay, H. Swedish primary healthcare practitioners’ perspectives on the impact of arts on prescription for patients and the wider society: a qualitative interview study. BMC Health Serv Res . (2021) 21:1277. doi: 10.1186/s12913-021-07258-7
35. Makin, S, and Gask, L. ‘Getting back to normal’: the added value of an art-based programme in promoting ‘recovery’ for common but chronic mental health problems. Chronic Illn . (2012) 8:64–75. doi: 10.1177/1742395311422613
36. Redmond, M, Sumner, RC, Crone, DM, and Hughes, S. ‘Light in dark places’: exploring qualitative data from a longitudinal study using creative arts as a form of social prescribing. Arts Health . (2019) 11:232–45. doi: 10.1080/17533015.2018.1490786
37. Stickley, T, and Eades, M. Arts on prescription: a qualitative outcomes study. Public Health . (2013) 127:727–34. doi: 10.1016/j.puhe.2013.05.001
38. Stickley, T, and Hui, A. Social prescribing through arts on prescription in a UK city: participants’ perspectives (part 1). Public Health . (2012) 126:574–9. doi: 10.1016/j.puhe.2012.04.002
39. Stickley, T, and Hui, A. Social prescribing through arts on prescription in a UK city, referrers’ perspective (part 2). J Public Health . (2012) 126:580–6. doi: 10.1016/j.puhe.2012.04.001
40. Baker, D, Ley, A, Alexander, J, and Beer, A. Eco art on prescription. Mental Health Soci Inclus . (2012) 16:84–9. doi: 10.1108/20428301211232496
41. Eades, G, and Ager, J. Time being: difficulties in integrating arts in health. J Royal Soci Promotion Health . (2008) 128:62–7. doi: 10.1177/1466424007087809
42. Golden, TL, Maier Lokuta, A, Mohanty, A, Tiedemann, A, Ng, TWC, Mendu, M, et al. Social prescription in the US: a pilot evaluation of mass cultural Council's “CultureRx”. Front Public Health . (2023) 10. doi: 10.3389/fpubh.2022.1016136
43. Hughes, S, Crone, DM, Sumner, RC, and Redmond, M. Understanding well-being outcomes in primary care arts on referral interventions: a mixed method study. Eur J Pers Cent Healthc . (2019) 7:1768.
44. Poulos, RG, Marwood, S, Harkin, D, Opher, S, Clift, S, Cole, AMD, et al. Arts on prescription for community-dwelling older people with a range of health and wellness needs. Health Soc Care Community . (2019) 27:483–92. doi: 10.1111/hsc.12669
45. Thomson, LJ, Morse, N, Elsden, E, and Chatterjee, HJ. Art, nature and mental health: assessing the biopsychosocial effects of a ‘creative green prescription’ museum programme involving horticulture, artmaking and collections. Perspect Public Health . (2020) 140:277–85. doi: 10.1177/1757913920910443
46. van de Venter, E, and Buller, AM. Arts on referral interventions: a mixed-methods study investigating factors associated with differential changes in mental well-being. J Public Health . (2015) 37:143–50. doi: 10.1093/pubmed/fdu028
47. Bergman, P, Rusaw, D, Bülow, PH, Skillmark, M, and Jansson, I. Effects of arts on prescription for persons with common mental disorders and/or musculoskeletal pain: a controlled study with 12 months follow-up. Cogent. Public Health . (2023) 10. doi: 10.1080/27707571.2023.2234631
48. Crone, DM, O’Connell, EE, Tyson, PJ, Clark-Stone, F, Opher, S, and James, DVB. ‘It helps me make sense of the world’: the role of an art intervention for promoting health and wellbeing in primary care—perspectives of patients, health professionals and artists. J Public Health . (2012) 20:519–24. doi: 10.1007/s10389-012-0495-x
49. Crone, DM, Sumner, RC, Baker, CM, Loughren, EA, Hughes, S, and James, DVB. “Artlift” arts-on-referral intervention in UK primary care: updated findings from an ongoing observational study. Eur J Pub Health . (2018) 28:404–9. doi: 10.1093/eurpub/cky021
50. Efstathopoulou, L, and Bungay, H. Mental health and resilience: arts on prescription for children and young people in a school setting. Public Health . (2021) 198:196–9. doi: 10.1016/j.puhe.2021.07.021
51. Holt, NJ. Tracking momentary experience in the evaluation of arts-on-prescription services: using mood changes during art workshops to predict global wellbeing change. Perspect Public Health . (2020) 140:270–6. doi: 10.1177/1757913920913060
52. Holt, NJ. The impact of remote arts on prescription: changes in mood, attention and loneliness during art workshops as mechanisms for wellbeing change. Nordic J Arts Culture Health . (2023) 5:1–13. doi: 10.18261/njach.5.1.1
53. Sumner, RC, Crone, DM, Baker, C, Hughes, S, Loughren, EA, and James, DVB. Factors associated with attendance, engagement and wellbeing change in an arts on prescription intervention. J Public Health . (2019). doi: 10.1093/pubmed/fdz032/5430503
54. Sumner, RC, Crone, DM, Hughes, S, and James, DVB. Arts on prescription: observed changes in anxiety, depression, and well-being across referral cycles. Public Health . (2021) 192:49–55. doi: 10.1016/j.puhe.2020.12.008
55. Tennant, R, Hiller, L, Fishwick, R, Platt, S, Joseph, S, Weich, S, et al. The Warwick-Edinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual Life Outcomes . (2007) 5:63. doi: 10.1186/1477-7525-5-63
56. Cajada, L, Stephenson, Z, and Bishopp, D. Exploring the psychometric properties of the resilience scale. Advers Resil Sci . (2023) 4:245–57. doi: 10.1007/s42844-023-00102-3
57. Campaign to End Loneliness. Measuring your impact on loneliness in later life (CtEL) . (2019).
58. Office for National Statistics (ONS). National indicators of loneliness . (2020). Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/compendium/nationalmeasurementofloneliness/2018/recommendednationalindicatorsofloneliness
59. Engeser, S, and Rheinberg, F. Flow, performance and moderators of challenge-skill balance. Motiv Emot . (2008) 32:158–72. doi: 10.1007/s11031-008-9102-4
60. Reissmann, A, Hauser, J, Stollberg, E, Kaunzinger, I, and Lange, KW. The role of loneliness in emerging adults’ everyday use of facebook–an experience sampling approach. Computers in Human Behav . (2018) 88:47–60. doi: 10.1016/j.chb.2018.06.011
61. Spitzer, RL, Kroenke, K, Williams, JBW, and Löwe, B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med . (2006) 166:1092. doi: 10.1001/archinte.166.10.1092
62. Kroenke, K, Spitzer, RL, Williams, JBW, and Löwe, B. An ultra-brief screening scale for anxiety and depression: The PHQ–4. Psychosomatics . (2009) 50:613–21. doi: 10.1176/appi.psy.50.6.613
63. Ericsson, A, Nyström, C, and Mannerkorpi, K. Psychometric properties of the Stress and Crisis Inventory (SCI-93) in females with fibromyalgia and chronic widespread pain. Nordic J Psychiatry . (2015) 69:28–34. doi: 10.3109/08039488.2014.915580
64. Zigmond, AS, and Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
65. Petticrew, M, and Roberts, H. Systematic reviews in the social sciences: A practical guide John Wiley & Sons (2008).
66. Braun, V, and Clark, V. Thematic analysis , Sage Publications Ltd (2021).
67. Jensen, A. Culture vitamins – an arts on prescription project in Denmark. Perspect Public Health . (2019) 139:131–6. doi: 10.1177/1757913919836145
68. Nyström, C, and Nyström, O. Stress and crisis inventory–SCI–93 . Partille: A process-oriented personality exploration starting from a crisis-theoretical frame of reference (1995).
69. Fairhall, N, Aggar, C, Kurrle, SE, Sherrington, C, Lord, S, Lockwood, K, et al. Frailty intervention trial (FIT). BMC Geriatr . (2008) 8:27. doi: 10.1186/1471-2318-8-27
70. Wilhelm, P, and Schoebi, D. Assessing mood in daily life. Eur J Psychol Assess . (2007) 23:258–67. doi: 10.1027/1015-5759.23.4.258
71. Thomson, L, and Chatterjee, H. UCL Museum wellbeing measures toolkit . Available at: https://www.ucl.ac.uk/culture/sites/culture/files/ucl_museum_wellbeing_measures_toolkit_sept2013.pdf
72. Daykin, N, Mansfield, L, Meads, C, Gray, K, Golding, A, Tomlinson, A, et al. The role of social capital in participatory arts for wellbeing: findings from a qualitative systematic review. Arts Health . (2021) 13:134–57. doi: 10.1080/17533015.2020.1802605
73. Maheswaran, H, Weich, S, Powell, J, and Stewart-Brown, S. Evaluating the responsiveness of the Warwick Edinburgh mental well-being scale (WEMWBS): group and individual level analysis. Health Qual Life Outcomes . (2012) 10:156. doi: 10.1186/1477-7525-10-156
74. Tomlinson, A, Lane, J, Julier, J, Duffy, LG, Payne, A, Mansfield, L, et al. A systematic review of the subjective wellbeing outcomes of engaging with visual arts for adults (“working-age”, 15–64 years) with diagnosed mental health conditions . London: (2018). Available at: https://whatworkswellbeing.org/wp-content/uploads/2020/01/Full-report-art-mental-health-wellbeing-Jan2018_0146725200.pdf
75. Jensen, A, and Bonde, LO. The use of arts interventions for mental health and wellbeing in health settings. Perspect Public Health . (2018) 138:209–14. doi: 10.1177/1757913918772602
76. Van Lith, T, Schofield, MJ, and Fenner, P. Identifying the evidence-base for art-based practices and their potential benefit for mental health recovery: a critical review. Disabil Rehabil . (2013) 35:1309–23. doi: 10.3109/09638288.2012.732188
77. Peterson, C, Park, N, and Seligman, MEP. Orientations to happiness and life satisfaction: the full life versus the empty life. J Happiness Stud . (2005) 6:25–41. doi: 10.1007/s10902-004-1278-z
78. Holt, NJ. Using the experience-sampling method to examine the psychological mechanisms by which participatory art improves wellbeing. Perspect Public Health . (2018) 138:55–65. doi: 10.1177/1757913917739041
79. Seligman, MEP. Flourish: A visionary new understanding of happiness and well-being Free Press (2011).
80. Ryan, RM, and Deci, EL. Self-determination theory: Basic psychological needs in motivation, development, and wellness . NY: New York. Guilford Press (2018).
81. Williams, E, Dingle, GA, Jetten, J, and Rowan, C. Identification with arts-based groups improves mental wellbeing in adults with chronic mental health conditions. J Appl Soc Psychol . (2019) 49:15–26. doi: 10.1111/jasp.12561
82. Tierney, S, Rowe, R, Connally, EL, Roberts, NW, Mahtani, KR, and Gorenberg, J. Community initiatives for well-being in the United Kingdom and their role in developing social capital and addressing loneliness: a scoping review. Lifestyle Med . (2024) 5. doi: 10.1002/lim2.98
83. Milligan, C, Neary, D, Payne, S, Hanratty, B, Irwin, P, and Dowrick, C. Older men and social activity: a scoping review of Men’s sheds and other gendered interventions. Ageing Soc . (2016) 36:895–923. doi: 10.1017/S0144686X14001524
84. Eaton, J, and Tieber, C. The effects of coloring on anxiety, mood, and perseverance. Art Ther . (2017) 34:42–6. doi: 10.1080/07421656.2016.1277113
85. James, C, Drake, JE, and Winner, E. Expression versus distraction. Empir Stud Arts . (2018) 36:162–79. doi: 10.1177/0276237417718423
86. Todd, C, Camic, PM, Lockyer, B, Thomson, LJM, and Chatterjee, HJ. Museum-based programs for socially isolated older adults: understanding what works. Health Place . (2017) 48:47–55. doi: 10.1016/j.healthplace.2017.08.005
87. Thomson, LJ, Lockyer, B, Camic, PM, and Chatterjee, HJ. Effects of a museum-based social prescription intervention on quantitative measures of psychological wellbeing in older adults. Perspect Public Health . (2018) 138:28–38. doi: 10.1177/1757913917737563
88. Helitzer, E, Clements-Cortes, A, and Moss, H. Group singing on social prescription: a scoping review. Music Med . (2022) 14:226–37. doi: 10.47513/mmd.v14i4.849
Keywords: arts on prescription, arts on referral, health, mental wellbeing, arts activities, social prescribing, culture on prescription, primary healthcare
Citation: Jensen A, Holt N, Honda S and Bungay H (2024) The impact of arts on prescription on individual health and wellbeing: a systematic review with meta-analysis. Front. Public Health . 12:1412306. doi: 10.3389/fpubh.2024.1412306
Received: 04 April 2024; Accepted: 26 June 2024; Published: 09 July 2024.
Reviewed by:
Copyright © 2024 Jensen, Holt, Honda and Bungay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita Jensen, [email protected]
† ORCID: Anita Jensen, https://orcid.org/0000-0002-4771-7768 Nicola Holt, https://orcid.org/0000-0003-4994-7214 Hilary Bungay, https://orcid.org/0000-0001-8202-4521
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- DOI: 10.7759/cureus.63550
- Corpus ID: 270886569
Systematic Review and Critical Appraisal of Cauda Equina Syndrome Management During Pregnancy
- Chinedu Egu , Samuel Akintunde , +3 authors Elie Najjar
- Published in Cureus 30 June 2024
46 References
Management of cauda equina syndrome during pregnancy and postpartum, risk of adverse fetal outcomes following nonobstetric surgery during gestation: a nationwide population-based analysis, spinal emergency surgery during pregnancy: contemporary strategies and outcome., the management of symptomatic lumbar disc herniation in pregnancy: a systematic review, methodological quality of case series studies: an introduction to the jbi critical appraisal tool, maternal admissions to a scottish intensive care unit, cauda equina syndrome in an obese pregnant patient secondary to double level lumbar disc herniation – a case report and review of literature, operative management of perinatal lumbar disc herniation and cauda equina syndrome: a case series, surgical intervention for cauda equina syndrome in the second and third trimesters of pregnancy: a report of three cases, lumbar disk herniation during pregnancy: a review on general management and timing of surgery, related papers.
Showing 1 through 3 of 0 Related Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Can Fam Physician
- v.39; 1993 May
Critical appraisal of review articles.
Review articles are an important source of clinical information for family physicians. However, the volume of available reviews is vast and their scientific quality varies enormously. Family physicians must be able to identify trustworthy reviews quickly. This article outlines practical and flexible guidelines for critical appraisal and discusses the respective roles of review articles and original research reports in guiding clinical practice.
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References .
Images in this article
p1099-a on p.1099
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Horwitz RI. Complexity and contradiction in clinical trial research. Am J Med. 1987 Mar; 82 (3):498–510. [ PubMed ] [ Google Scholar ]
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985 Mar-Apr; 27 (5):335–371. [ PubMed ] [ Google Scholar ]
- Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987 Feb 19; 316 (8):450–455. [ PubMed ] [ Google Scholar ]
- Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987 Mar; 106 (3):485–488. [ PubMed ] [ Google Scholar ]
- Oxman AD, Guyatt GH. Guidelines for reading literature reviews. CMAJ. 1988 Apr 15; 138 (8):697–703. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mulrow CD, Thacker SB, Pugh JA. A proposal for more informative abstracts of review articles. Ann Intern Med. 1988 Apr; 108 (4):613–615. [ PubMed ] [ Google Scholar ]
- Haynes RB, Mulrow CD, Huth EJ, Altman DG, Gardner MJ. More informative abstracts revisited. Ann Intern Med. 1990 Jul 1; 113 (1):69–76. [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Critical appraisal tools and reporting guidelines are the two most important instruments available to researchers and practitioners involved in research, evidence-based practice, and policymaking. Each of these instruments has unique characteristics, and both instruments play an essential role in evidence-based practice and decision-making.
Critical appraisal of scientific literature is an important skill to be mastered not only by academic medical professionals but also by those involved in clinical practice. Before incorporating changes into the management of their patients, a thorough evaluation of the current or published literature is an important step in clinical practice.
In the context of a literature search, critical appraisal is the process of systematically evaluating and assessing the research you have found in order to determine its quality and validity. It is essential to evidence-based practice. More formally, critical appraisal is a systematic evaluation of research papers in order to answer the ...
Each participant was asked to appraise three analytic studies, two descriptive studies and one literature review, using the appropriate critical appraisal tool as identified by the participant.
A formal literature review is an evidence-based, in-depth analysis of a subject. There are many reasons for writing one and these will influence the length and style of your review, but in essence a literature review is a critical appraisal of the current collective knowledge on a subject. Rather than just being an exhaustive list of all that ...
Critical appraisal is the course of action for watchfully and systematically examining research to assess its reliability, value and relevance in order to direct professionals in their vital clinical decision making [ 1 ]. Critical appraisal is essential to: Continuing Professional Development (CPD).
The purpose of the current article is to define critical appraisal, identify its benefits, discuss conceptual issues influencing the adequacy of a critical appraisal, and detail procedures to help reviewers undertake critical appraisals. A critical appraisal involves a careful and systematic assessment of a study's trustworthiness or ...
JBI Critical appraisal tools have been developed by the JBI and collaborators and approved by the JBI Scientific Committee following extensive peer review. Although designed for use in systematic reviews, JBI critical appraisal tools can also be used when creating Critically Appraised Topics (CAT), in journal clubs and as an educational tool.
Critical appraisal of research papers is a component of everyday academic life, whether as a student as part of an assignment, as a researcher as part of a literature review or as a teacher preparing a lecture. Learn more from this post.
Critical appraisal is the process of carefully, judiciously and systematically examining research to adjudicate its trustworthiness and its value and relevance in clinical practice.
Appraise Your Research Articles The structure of a literature review should include the following: An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review, Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches entirely], An ...
Documenting critical appraisal decisions. As you closely examine full articles, you will be making judgements about why to include or exclude each study from your review. Documenting your reasoning will help you reassure yourself and demonstrate to others that you have been systematic and unbiased in your appraisal decisions.
This Review article presents a 10-step guide to the critical appraisal of research literature to assist clinicians with the task of identifying the most relevant, high-quality studies available to ...
Critical Appraisal Some reviews require a critical appraisal for each study that makes it through the screening process. This involves a risk of bias assessment and/or a quality assessment. The goal of these reviews is not just to find all of the studies, but to determine their methodological rigor, and therefore, their credibility. "Critical appraisal is the balanced assessment of a piece of ...
Critical appraisal is the assessment of research studies' worth to clinical practice. Critical appraisal—the heart of evidence-based practice—involves four phases: rapid critical appraisal, evaluation, synthesis, and recommendation. This article reviews each phase and provides examples, tips, and caveats to help evidence appraisers ...
Tools for Critical Appraisal of Studies. "The purpose of critical appraisal is to determine the scientific merit of a research report and its applicability to clinical decision making."1 Conducting a critical appraisal of a study is imperative to any well executed evidence review, but the process can be time consuming and difficult.2 The ...
Why is critical appraisal needed? Literature searches using databases like Medline or EMBASE often result in an overwhelming volume of results which can vary in quality. Similarly, those who browse medical literature for the purposes of CPD or in response to a clinical query will know that there are vast amounts of content available. Critical appraisal helps to reduce the burden and allow you ...
JBI's critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers. These tools have been revised. Recently published articles detail the revision. "Assessing the risk of bias of quantitative analytical studies: introducing the vision for critical appraisal within JBI systematic reviews".
Critical appraisal refers to the process of judging the validity and quality of a research paper. Because your review will be a synthesis of the research conducted by others, it is important to consider major points about the studies you include such as: Is the study valid?
Critical appraisal is the systematic evaluation of clinical research papers that helps us establish if the results are valid and if they could be used to inform medical decision in a given local population and context. There are several published guidelines for critically appraising the scientific literature, most of which are structured as ...
Checklist Given the surge in poor-quality systematic review publications, we sought to describe a checklist of seven practical tips from the authors' collective experience of writing and critically appraising systematic reviews, hoping that they will assist busy clinicians to critically appraise systematic reviews both as manuscript reviewers and as readers and research users.
Data synthesis: A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant original research, and to collect and analyze data from the studies that are included in the review. Critical appraisal methods address both the credibility (quality of ...
Critical appraisal is the process of systematically evaluating the methodology and results of research studies in terms of validity, value and relevance. This course will be a great place to start for anyone who wants to learn more about critical appraisal of the literature - for any purpose, even purposes you hadn't thought of yet!
existing literature on a particular topic of interest. Critical reviews take a reflective look at the research that has been done in a particular area of interest and assess its credibility by using appraisal instruments or critical interpretive methods. Critical reviews attempt to constructively inform other scholars about the
Ventilator-associated pneumonia (VAP) is a prevalent and grave hospital-acquired infection that affects mechanically ventilated patients. Diverse diagnostic criteria can significantly affect VAP research by complicating the identification and management of the condition, which may also impact clinical management. We conducted this review to assess the diagnostic criteria and the definitions of ...
This paper reports on a comprehensive literature review using the Systematic Literature Review approach to improve agility and flexibility at the Sustainable Supply Chain Management (SSCM) level. T...
This review aims to address knowledge gaps in the 2020 consensus on CACs of major European medical associations, considering novel evidence on critical issues in both pre- and in-hospital OHCA management, such as the timing of coronary angiography and the use of extracorporeal cardiopulmonary resuscitation (eCPR).
The review used PRISMA reporting structures. Critical Appraisal Skills Programme (CASP) templates were used for qualitative and quantitative studies, and the Mixed Methods Appraisal Tool (MMAT) for studies with a mixed methods protocol to assess quality and risk of bias. A narrative review of the qualitative data was conducted.
Semantic Scholar extracted view of "Systematic Review and Critical Appraisal of Cauda Equina Syndrome Management During Pregnancy" by Chinedu Egu et al.
Abstract. Review articles are an important source of clinical information for family physicians. However, the volume of available reviews is vast and their scientific quality varies enormously. Family physicians must be able to identify trustworthy reviews quickly. This article outlines practical and flexible guidelines for critical appraisal ...