- Second Opinion
- Research & Innovation
- Patients & Families
- Health Professionals
- Recently Visited
- Segunda opinión
- Refer a patient
- MyChart Login

Healthier, Happy Lives Blog
Sort articles by..., sort by category.
- Celebrating Volunteers
- Community Outreach
- Construction Updates
- Family-Centered Care
- Healthy Eating
- Heart Center
- Interesting Things
- Mental Health
- Patient Stories
- Research and Innovation
- Safety Tips
- Sustainability
- World-Class Care
About Our Blog
- Back-to-School
- Pediatric Technology
Latest Posts
- Honoring the ‘Miracle Days’ of Transplant Patients
- Summer Smiles: Expert Tips for a Positive Summer and Stronger Families
- Transplant Recipient Celebrates Impressive 40-Year ‘Heart-Aversary’ in a Remarkable Year for Stanford
- 25 Years of Exceptional Care for Moms and Babies in Redwood City
- Remembering Willie Mays

Health Hazards of Homework
March 18, 2014 | Julie Greicius Pediatrics .

A new study by the Stanford Graduate School of Education and colleagues found that students in high-performing schools who did excessive hours of homework “experienced greater behavioral engagement in school but also more academic stress, physical health problems, and lack of balance in their lives.”
Those health problems ranged from stress, headaches, exhaustion, sleep deprivation, weight loss and stomach problems, to psycho-social effects like dropping activities, not seeing friends or family, and not pursuing hobbies they enjoy.
In the Stanford Report story about the research, Denise Pope , a senior lecturer at the Stanford Graduate School of Education and a co-author of the study published in the Journal of Experimental Education , says, “Our findings on the effects of homework challenge the traditional assumption that homework is inherently good.”
The study was based on survey data from a sample of 4,317 students from 10 high-performing high schools in California communities in which median household income exceeded $90,000. Of the students surveyed, homework volume averaged about 3.1 hours each night.
“It is time to re-evaluate how the school environment is preparing our high school student for today’s workplace,” says Neville Golden, MD , chief of adolescent medicine at Stanford Medicine Children’s Health and a professor at the School of Medicine. “This landmark study shows that excessive homework is counterproductive, leading to sleep deprivation, school stress and other health problems. Parents can best support their children in these demanding academic environments by advocating for them through direct communication with teachers and school administrators about homework load.”
Related Posts

Top-ranked group group in Los Gatos, Calif., is now a part of one of the…

The Stanford Medicine Children’s Health network continues to grow with our newest addition, Town and…
- Julie Greicius
- more by this author...
Connect with us:
Download our App:
ABOUT STANFORD MEDICINE CHILDREN'S HEALTH
- Leadership Team
- Vision, Mission & Values
- The Stanford Advantage
- Government and Community Relations
LUCILE PACKARD FOUNDATION FOR CHILDREN'S HEALTH
- Get Involved
- Volunteering Services
- Auxiliaries & Affiliates
- Our Hospital
- Send a Greeting Card
- New Hospital
- Refer a Patient
- Pay Your Bill

Also Find Us on:
- Notice of Nondiscrimination
- Terms of Use
- Privacy Policy
- Code of Conduct
- Price Transparency
- Stanford School of Medicine
- Stanford Health Care
- Stanford University

Homework Struggles May Not Be a Behavior Problem
Exploring some options to understand and help..
Posted August 2, 2022 | Reviewed by Abigail Fagan
- Mental health challenges and neurodevelopmental differences directly affect children's ability to do homework.
- Understanding what difficulties are getting in the way—beyond the usual explanation of a behavior problem—is key.
- Sleep and mental health needs can take priority over homework completion.
Chelsea was in 10th grade the first time I told her directly to stop doing her homework and get some sleep. I had been working with her since she was in middle school, treating her anxiety disorder. She deeply feared disappointing anyone—especially her teachers—and spent hours trying to finish homework perfectly. The more tired and anxious she got, the harder it got for her to finish the assignments.

One night Chelsea called me in despair, feeling hopeless. She was exhausted and couldn’t think straight. She felt like a failure and that she was a burden to everyone because she couldn’t finish her homework.
She was shocked when I told her that my prescription for her was to go to sleep now—not to figure out how to finish her work. I told her to leave her homework incomplete and go to sleep. We briefly discussed how we would figure it out the next day, with her mom and her teachers. At that moment, it clicked for her that it was futile to keep working—because nothing was getting done.
This was an inflection point for her awareness of when she was emotionally over-cooked and when she needed to stop and take a break or get some sleep. We repeated versions of this phone call several times over the course of her high school and college years, but she got much better at being able to do this for herself most of the time.
When Mental Health Symptoms Interfere with Homework
Kids with mental health or neurodevelopmental challenges often struggle mightily with homework. Challenges can come up in every step of the homework process, including, but not limited to:
- Remembering and tracking assignments and materials
- Getting the mental energy/organization to start homework
- Filtering distractions enough to persist with assignments
- Understanding unspoken or implied parts of the homework
- Remembering to bring finished homework to class
- Being in class long enough to know the material
- Tolerating the fear of not knowing or failing
- Not giving up the assignment because of a panic attack
- Tolerating frustration—such as not understanding—without emotional dysregulation
- Being able to ask for help—from a peer or a teacher and not being afraid to reach out
This list is hardly comprehensive. ADHD , autism spectrum disorder, social anxiety , generalized anxiety, panic disorder, depression , dysregulation, and a range of other neurodevelopmental and mental health challenges cause numerous learning differences and symptoms that can specifically and frequently interfere with getting homework done.

The Usual Diagnosis for Homework Problems is "Not Trying Hard Enough"
Unfortunately, when kids frequently struggle to meet homework demands, teachers and parents typically default to one explanation of the problem: The child is making a choice not to do their homework. That is the default “diagnosis” in classrooms and living rooms. And once this framework is drawn, the student is often seen as not trying hard enough, disrespectful, manipulative, or just plain lazy.
The fundamental disconnect here is that the diagnosis of homework struggles as a behavioral choice is, in fact, only one explanation, while there are so many other diagnoses and differences that impair children's ability to consistently do their homework. If we are trying to create solutions based on only one understanding of the problem, the solutions will not work. More devastatingly, the wrong solutions can worsen the child’s mental health and their long-term engagement with school and learning.
To be clear, we aren’t talking about children who sometimes struggle with or skip homework—kids who can change and adapt their behaviors and patterns in response to the outcomes of that struggle. For this discussion, we are talking about children with mental health and/or neurodevelopmental symptoms and challenges that create chronic difficulties with meeting homework demands.
How Can You Help a Child Who Struggles with Homework?
How can you help your child who is struggling to meet homework demands because of their ADHD, depression, anxiety, OCD , school avoidance, or any other neurodevelopmental or mental health differences? Let’s break this down into two broad areas—things you can do at home, and things you can do in communication with the school.

Helping at Home
The following suggestions for managing school demands at home can feel counterintuitive to parents—because we usually focus on helping our kids to complete their tasks. But mental health needs jump the line ahead of task completion. And starting at home will be key to developing an idea of what needs to change at school.
- Set an end time in the evening after which no more homework will be attempted. Kids need time to decompress and they need sleep—and pushing homework too close to or past bedtime doesn’t serve their educational needs. Even if your child hasn’t been able to approach the homework at all, even if they have avoided and argued the whole evening, it is still important for everyone to have a predictable time to shut down the whole process.
- If there are arguments almost every night about homework, if your child isn’t starting homework or finishing it, reframe it from failure into information. It’s data to put into problem-solving. We need to consider other possible explanations besides “behavioral choice” when trying to understand the problem and create effective solutions. What problems are getting in the way of our child’s meeting homework demands that their peers are meeting most of the time?
- Try not to argue about homework. If you can check your own anxiety and frustration, it can be more productive to ally with your child and be curious with them. Kids usually can’t tell you a clear “why” but maybe they can tell you how they are feeling and what they are thinking. And if your child can’t talk about it or just keeps saying “I don't know,” try not to push. Come back another time. Rushing, forcing, yelling, and threatening will predictably not help kids do homework.

Helping at School
The second area to explore when your neurodiverse child struggles frequently with homework is building communication and connections with school and teachers. Some places to focus on include the following.
- Label your child’s diagnoses and break down specific symptoms for the teachers and school team. Nonjudgmental, but specific language is essential for teachers to understand your child’s struggles. Breaking their challenges down into the problems specific to homework can help with building solutions. As your child gets older, help them identify their difficulties and communicate them to teachers.
- Let teachers and the school team know that your child’s mental health needs—including sleep—take priority over finishing homework. If your child is always struggling to complete homework and get enough sleep, or if completing homework is leading to emotional meltdowns every night, adjusting their homework demands will be more successful than continuing to push them into sleep deprivation or meltdowns.
- Request a child study team evaluation to determine if your child qualifies for services under special education law such as an IEP, or accommodations through section 504—and be sure that homework adjustments are included in any plan. Or if such a plan is already in place, be clear that modification of homework expectations needs to be part of it.
The Long-Term Story
I still work with Chelsea and she recently mentioned how those conversations so many years ago are still part of how she approaches work tasks or other demands that are spiking her anxiety when she finds herself in a vortex of distress. She stops what she is doing and prioritizes reducing her anxiety—whether it’s a break during her day or an ending to the task for the evening. She sees that this is crucial to managing her anxiety in her life and still succeeding at what she is doing.
Task completion at all costs is not a solution for kids with emotional needs. Her story (and the story of many of my patients) make this crystal clear.

Candida Fink, M.D. , is board certified in child/adolescent and general psychiatry. She practices in New York and has co-authored two books— The Ups and Downs of Raising a Bipolar Child and Bipolar Disorder for Dummies.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

Sticking up for yourself is no easy task. But there are concrete skills you can use to hone your assertiveness and advocate for yourself.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience

Is homework a necessary evil?
After decades of debate, researchers are still sorting out the truth about homework’s pros and cons. One point they can agree on: Quality assignments matter.
By Kirsten Weir
March 2016, Vol 47, No. 3
Print version: page 36

- Schools and Classrooms
Homework battles have raged for decades. For as long as kids have been whining about doing their homework, parents and education reformers have complained that homework's benefits are dubious. Meanwhile many teachers argue that take-home lessons are key to helping students learn. Now, as schools are shifting to the new (and hotly debated) Common Core curriculum standards, educators, administrators and researchers are turning a fresh eye toward the question of homework's value.
But when it comes to deciphering the research literature on the subject, homework is anything but an open book.
The 10-minute rule
In many ways, homework seems like common sense. Spend more time practicing multiplication or studying Spanish vocabulary and you should get better at math or Spanish. But it may not be that simple.
Homework can indeed produce academic benefits, such as increased understanding and retention of the material, says Duke University social psychologist Harris Cooper, PhD, one of the nation's leading homework researchers. But not all students benefit. In a review of studies published from 1987 to 2003, Cooper and his colleagues found that homework was linked to better test scores in high school and, to a lesser degree, in middle school. Yet they found only faint evidence that homework provided academic benefit in elementary school ( Review of Educational Research , 2006).
Then again, test scores aren't everything. Homework proponents also cite the nonacademic advantages it might confer, such as the development of personal responsibility, good study habits and time-management skills. But as to hard evidence of those benefits, "the jury is still out," says Mollie Galloway, PhD, associate professor of educational leadership at Lewis & Clark College in Portland, Oregon. "I think there's a focus on assigning homework because [teachers] think it has these positive outcomes for study skills and habits. But we don't know for sure that's the case."
Even when homework is helpful, there can be too much of a good thing. "There is a limit to how much kids can benefit from home study," Cooper says. He agrees with an oft-cited rule of thumb that students should do no more than 10 minutes a night per grade level — from about 10 minutes in first grade up to a maximum of about two hours in high school. Both the National Education Association and National Parent Teacher Association support that limit.
Beyond that point, kids don't absorb much useful information, Cooper says. In fact, too much homework can do more harm than good. Researchers have cited drawbacks, including boredom and burnout toward academic material, less time for family and extracurricular activities, lack of sleep and increased stress.
In a recent study of Spanish students, Rubén Fernández-Alonso, PhD, and colleagues found that students who were regularly assigned math and science homework scored higher on standardized tests. But when kids reported having more than 90 to 100 minutes of homework per day, scores declined ( Journal of Educational Psychology , 2015).
"At all grade levels, doing other things after school can have positive effects," Cooper says. "To the extent that homework denies access to other leisure and community activities, it's not serving the child's best interest."
Children of all ages need down time in order to thrive, says Denise Pope, PhD, a professor of education at Stanford University and a co-founder of Challenge Success, a program that partners with secondary schools to implement policies that improve students' academic engagement and well-being.
"Little kids and big kids need unstructured time for play each day," she says. Certainly, time for physical activity is important for kids' health and well-being. But even time spent on social media can help give busy kids' brains a break, she says.
All over the map
But are teachers sticking to the 10-minute rule? Studies attempting to quantify time spent on homework are all over the map, in part because of wide variations in methodology, Pope says.
A 2014 report by the Brookings Institution examined the question of homework, comparing data from a variety of sources. That report cited findings from a 2012 survey of first-year college students in which 38.4 percent reported spending six hours or more per week on homework during their last year of high school. That was down from 49.5 percent in 1986 ( The Brown Center Report on American Education , 2014).
The Brookings report also explored survey data from the National Assessment of Educational Progress, which asked 9-, 13- and 17-year-old students how much homework they'd done the previous night. They found that between 1984 and 2012, there was a slight increase in homework for 9-year-olds, but homework amounts for 13- and 17-year-olds stayed roughly the same, or even decreased slightly.
Yet other evidence suggests that some kids might be taking home much more work than they can handle. Robert Pressman, PhD, and colleagues recently investigated the 10-minute rule among more than 1,100 students, and found that elementary-school kids were receiving up to three times as much homework as recommended. As homework load increased, so did family stress, the researchers found ( American Journal of Family Therapy , 2015).
Many high school students also seem to be exceeding the recommended amounts of homework. Pope and Galloway recently surveyed more than 4,300 students from 10 high-achieving high schools. Students reported bringing home an average of just over three hours of homework nightly ( Journal of Experiential Education , 2013).
On the positive side, students who spent more time on homework in that study did report being more behaviorally engaged in school — for instance, giving more effort and paying more attention in class, Galloway says. But they were not more invested in the homework itself. They also reported greater academic stress and less time to balance family, friends and extracurricular activities. They experienced more physical health problems as well, such as headaches, stomach troubles and sleep deprivation. "Three hours per night is too much," Galloway says.
In the high-achieving schools Pope and Galloway studied, more than 90 percent of the students go on to college. There's often intense pressure to succeed academically, from both parents and peers. On top of that, kids in these communities are often overloaded with extracurricular activities, including sports and clubs. "They're very busy," Pope says. "Some kids have up to 40 hours a week — a full-time job's worth — of extracurricular activities." And homework is yet one more commitment on top of all the others.
"Homework has perennially acted as a source of stress for students, so that piece of it is not new," Galloway says. "But especially in upper-middle-class communities, where the focus is on getting ahead, I think the pressure on students has been ratcheted up."
Yet homework can be a problem at the other end of the socioeconomic spectrum as well. Kids from wealthier homes are more likely to have resources such as computers, Internet connections, dedicated areas to do schoolwork and parents who tend to be more educated and more available to help them with tricky assignments. Kids from disadvantaged homes are more likely to work at afterschool jobs, or to be home without supervision in the evenings while their parents work multiple jobs, says Lea Theodore, PhD, a professor of school psychology at the College of William and Mary in Williamsburg, Virginia. They are less likely to have computers or a quiet place to do homework in peace.
"Homework can highlight those inequities," she says.
Quantity vs. quality
One point researchers agree on is that for all students, homework quality matters. But too many kids are feeling a lack of engagement with their take-home assignments, many experts say. In Pope and Galloway's research, only 20 percent to 30 percent of students said they felt their homework was useful or meaningful.
"Students are assigned a lot of busywork. They're naming it as a primary stressor, but they don't feel it's supporting their learning," Galloway says.
"Homework that's busywork is not good for anyone," Cooper agrees. Still, he says, different subjects call for different kinds of assignments. "Things like vocabulary and spelling are learned through practice. Other kinds of courses require more integration of material and drawing on different skills."
But critics say those skills can be developed with many fewer hours of homework each week. Why assign 50 math problems, Pope asks, when 10 would be just as constructive? One Advanced Placement biology teacher she worked with through Challenge Success experimented with cutting his homework assignments by a third, and then by half. "Test scores didn't go down," she says. "You can have a rigorous course and not have a crazy homework load."
Still, changing the culture of homework won't be easy. Teachers-to-be get little instruction in homework during their training, Pope says. And despite some vocal parents arguing that kids bring home too much homework, many others get nervous if they think their child doesn't have enough. "Teachers feel pressured to give homework because parents expect it to come home," says Galloway. "When it doesn't, there's this idea that the school might not be doing its job."
Galloway argues teachers and school administrators need to set clear goals when it comes to homework — and parents and students should be in on the discussion, too. "It should be a broader conversation within the community, asking what's the purpose of homework? Why are we giving it? Who is it serving? Who is it not serving?"
Until schools and communities agree to take a hard look at those questions, those backpacks full of take-home assignments will probably keep stirring up more feelings than facts.
Further reading
- Cooper, H., Robinson, J. C., & Patall, E. A. (2006). Does homework improve academic achievement? A synthesis of research, 1987-2003. Review of Educational Research, 76 (1), 1–62. doi: 10.3102/00346543076001001
- Galloway, M., Connor, J., & Pope, D. (2013). Nonacademic effects of homework in privileged, high-performing high schools. The Journal of Experimental Education, 81 (4), 490–510. doi: 10.1080/00220973.2012.745469
- Pope, D., Brown, M., & Miles, S. (2015). Overloaded and underprepared: Strategies for stronger schools and healthy, successful kids . San Francisco, CA: Jossey-Bass.
Letters to the Editor
- Send us a letter
- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
School Stress Takes A Toll On Health, Teens And Parents Say
Patti Neighmond

Colleen Frainey, 16, of Tualatin, Ore., cut back on advanced placement classes in her junior year because the stress was making her physically ill. Toni Greaves for NPR hide caption
Colleen Frainey, 16, of Tualatin, Ore., cut back on advanced placement classes in her junior year because the stress was making her physically ill.
When high school junior Nora Huynh got her report card, she was devastated to see that she didn't get a perfect 4.0.
Nora "had a total meltdown, cried for hours," her mother, Jennie Huynh of Alameda, Calif., says. "I couldn't believe her reaction."
Nora is doing college-level work, her mother says, but many of her friends are taking enough advanced classes to boost their grade-point averages above 4.0. "It breaks my heart to see her upset when she's doing so awesome and going above and beyond."
And the pressure is taking a physical toll, too. At age 16, Nora is tired, is increasingly irritated with her siblings and often suffers headaches, her mother says.
Teens Talk Stress
When NPR asked on Facebook if stress is an issue for teenagers, they spoke loud and clear:
- "Academic stress has been a part of my life ever since I can remember," wrote Bretta McCall, 16, of Seattle. "This year I spend about 12 hours a day on schoolwork. I'm home right now because I was feeling so sick from stress I couldn't be at school. So as you can tell, it's a big part of my life!"
- "At the time of writing this, my weekend assignments include two papers, a PowerPoint to go with a 10-minute presentation, studying for a test and two quizzes, and an entire chapter (approximately 40 pages) of notes in a college textbook," wrote Connor West of New Jersey.
- "It's a problem that's basically brushed off by most people," wrote Kelly Farrell in Delaware. "There's this mentality of, 'You're doing well, so why are you complaining?' " She says she started experiencing symptoms of stress in middle school, and was diagnosed with panic disorder and generalized anxiety disorder in high school.
- "Parents are the worst about all of this," writes Colin Hughes of Illinois. "All I hear is, 'Work harder, you're a smart kid, I know you have it in you, and if you want to go to college you need to work harder.' It's a pain."
Parents are right to be worried about stress and their children's health, says Mary Alvord , a clinical psychologist in Maryland and public education coordinator for the American Psychological Association.
"A little stress is a good thing," Alvord says. "It can motivate students to be organized. But too much stress can backfire."
Almost 40 percent of parents say their high-schooler is experiencing a lot of stress from school, according to a new NPR poll conducted with the Robert Wood Johnson Foundation and the Harvard School of Public Health. In most cases, that stress is from academics, not social issues or bullying, the poll found. (See the full results here .)
Homework was a leading cause of stress, with 24 percent of parents saying it's an issue.
Teenagers say they're suffering, too. A survey by the American Psychological Association found that nearly half of all teens — 45 percent — said they were stressed by school pressures.
Chronic stress can cause a sense of panic and paralysis, Alvord says. The child feels stuck, which only adds to the feeling of stress.
Parents can help put the child's distress in perspective, particularly when they get into what Alvord calls catastrophic "what if" thinking: "What if I get a bad grade, then what if that means I fail the course, then I'll never get into college."
Then move beyond talking and do something about it.

Colleen pets her horse, Bishop. They had been missing out on rides together because of homework. Toni Greaves for NPR hide caption
Colleen pets her horse, Bishop. They had been missing out on rides together because of homework.
That's what 16-year-old Colleen Frainey of Tualatin, Ore., did. As a sophomore last year, she was taking all advanced courses. The pressure was making her sick. "I didn't feel good, and when I didn't feel good I felt like I couldn't do my work, which would stress me out more," she says.
Mom Abigail Frainey says, "It was more than we could handle as a family."
With encouragement from her parents, Colleen dropped one of her advanced courses. The family's decision generated disbelief from other parents. "Why would I let her take the easy way out?" Abigail Frainey heard.
But she says dialing down on academics was absolutely the right decision for her child. Colleen no longer suffers headaches or stomachaches. She's still in honors courses, but the workload this year is manageable.
Even better, Colleen now has time to do things she never would have considered last year, like going out to dinner with the family on a weeknight, or going to the barn to ride her horse, Bishop.
Psychologist Alvord says a balanced life should be the goal for all families. If a child is having trouble getting things done, parents can help plan the week, deciding what's important and what's optional. "Just basic time management — that will help reduce the stress."
- Children's Health
Opinion | Social-Emotional Learning
If we’re serious about student well-being, we must change the systems students learn in, here are five steps high schools can take to support students' mental health., by tim klein and belle liang oct 14, 2022.

Shutterstock / SvetaZi
This article is part of the collection: Making Sense of K-12 Student Mental Health.
Educators and parents started this school year with bated breath. Last year’s stress led to record levels of teacher burnout and mental health challenges for students.
Even before the pandemic, a mental health crisis among high schoolers loomed. According to a survey administered by the Centers for Disease Control and Prevention in 2019, 37 percent of high school students said they experienced persistent sadness or hopelessness and 19 percent reported suicidality. In response, more than half of all U.S. states mandated that schools have a mental health curriculum or include mental health in their standards .
As mental health professionals and co-authors of a book about the pressure and stress facing high school students, we’ve spent our entire careers supporting students’ mental health. Traditionally, mental health interventions are individualized and they focus on helping students manage and change their behaviors to cope with challenges they’re facing. But while working with schools and colleges across the globe as we conducted research for our book , we realized that most interventions don’t address systemic issues causing mental health problems in the first place.
It’s time we acknowledge that our education systems are directly contributing to the youth mental health crisis. And if we are serious about student well-being, we must change the systems they learn in.
Here are five bold steps that high schools can take to boost mental health.
Limit Homework or Make it Optional
Imagine applying for a job, and the hiring manager informs you that in addition to a full workday in the office, you’ll be assigned three more hours of work every night. Does this sound like a healthy work-life balance? Most adults would consider this expectation ridiculous and unsustainable. Yet, this is the workload most schools place on high school students.
Research shows that excessive homework leads to increased stress, physical health problems and a lack of balance in students' lives. And studies have shown that more than two hours of daily homework can be counterproductive , yet many teachers assign more.
Homework proponents argue that homework improves academic performance. Indeed, a meta-analysis of research on this issue found a correlation between homework and achievement. But correlation isn’t causation. Does homework cause achievement or do high achievers do more homework? While it’s likely that homework completion signals student engagement, which in turn leads to academic achievement, there’s little evidence to suggest that homework itself improves engagement in learning.
Another common argument is that homework helps students develop skills related to problem-solving, time-management and self-direction. But these skills can be explicitly taught during the school day rather than after school.
Limiting homework or moving to an optional homework policy not only supports student well-being, but it can also create a more equitable learning environment. According to the American Psychological Association, students from more affluent families are more likely to have access to resources such as devices, internet, dedicated work space and the support necessary to complete their work successfully—and homework can highlight those inequities .
Whether a school limits homework or makes it optional, it’s critical to remember that more important than the amount of homework assigned, is designing the type of activities that engage students in learning. When students are intrinsically motivated to do their homework, they are more engaged in the work, which in turn is associated with academic achievement.
Cap the Number of APs Students Can Take
Advanced Placement courses give students a taste of college-level work and, in theory, allow them to earn college credits early. Getting good grades on AP exams is associated with higher GPAs in high school and success in college, but the research tends to be correlational rather than causational.
In 2008, a little over 180,000 students took three or more AP exams. By 2018, that number had ballooned to almost 350,000 students .
However, this expansion has come at the expense of student well-being.
Over the years, we’ve heard many students express that they feel pressure to take as many AP classes as possible, which overloads them with work. That’s troubling because studies show that students who take AP classes and exams are twice as likely to report adverse physical and emotional health .
AP courses and exams also raise complex issues of equity. In 2019, two out of three Harvard freshmen reported taking AP Calculus in high school, according to Jeff Selingo, author of “ Who Gets In and Why: A Year Inside College Admissions ,” yet only half of all high schools in the country offer the course. And opportunity gaps exist for advanced coursework such as AP courses and dual enrollment, with inequitable distribution of funding and support impacting which students are enrolling and experiencing success. According to the Center for American Progress, “National data from the Civil Rights Data Collection show that students who are Black, Indigenous, and other non-Black people of color (BIPOC) are not enrolled in AP courses at rates comparable to their white and Asian peers and experience less success when they are—and the analysis for this report finds this to be true even when they attend schools with similar levels of AP course availability.”
Limiting the number of AP courses students take can protect mental health and create a more equitable experience for students.
Eliminate Class Rankings
In a study we conducted about mental health problems among high school girls, we found that a primary driver of stress was their perception of school as a hypercompetitive, zero-sum game where pervasive peer pressure to perform reigns supreme.
Class rankings fuel these cutthroat environments. They send a toxic message to young people: success requires doing better than your peers.
Ranking systems help highly selective colleges decide which students to admit or reject for admission. The purpose of high school is to develop students to their own full potential, rather than causing them to fixate on measuring up to others. Research shows that ranking systems undercut students’ learning and damage social relationships by turning peers into opponents.
Eliminating class rankings sends a powerful message to students that they are more than a number.
Become an Admission Test Objector
COVID-19 ushered in the era of test-optional admissions. De-centering standardized tests in the college application process is unequivocally a good thing. Standardized tests don’t predict student success in college , they only widen the achievement gap between privileged and underprivileged students and damage students' mental health .
Going “test optional” is an excellent first step, but it's not enough.
Even as more colleges have made tests optional, affluent students submit test scores at a higher rate than their lower-income peers and are admitted at higher rates , suggesting that testing still gives them an edge.
High schools must adhere to standardized test mandates, but they don’t have to endorse them. They can become test objectors by publicly proclaiming that these tests hold no inherent value. They can stop teaching to the test and educate parents on why they are doing so. Counseling departments can inform colleges that their school is a test objector so admission teams won’t penalize students.
Of course, students and families will still find ways to wield these tests as a competitive advantage. Over time, the more schools and educators unite to denounce these tests, the less power they will hold over students and families.
Big change starts with small steps.
Stand For What You Value
Critics may argue that such policies might hurt student outcomes. How will colleges evaluate school rigor if we limit AP courses and homework? How will students demonstrate their merits without class rankings and standardized test scores?
The truth is, the best school systems in the world succeed without homework, standardized test scores or an obsession with rigorous courses. And many U.S. schools have found creative and empowering ways to showcase student merit beyond rankings and test scores.
If we aren’t willing to change policies and practices that have been shown to harm students’ well-being, we have to ask ourselves: Do we really value mental health?
Thankfully, it doesn’t have to be an either/or scenario: We can design school systems that help students thrive academically and psychologically.
Belle Liang and Tim Klein are mental health professionals and co-authors of “How To Navigate Life: The New Science of Finding Your Way in School, Career and Life.”
Making Sense of K-12 Student Mental Health

Tackling the Youth Mental Health Crisis Head-On
By aaliyah a. samuel.

Adolescents Need More Proactive, Preventative Mental Health Supports in School
By sara potler lahayne.

Coronavirus
Kids’ mental health is in crisis. schools can get them help through a $1 billion fund, by nadia tamez-robledo.

When Student Anxiety Gets in the Way of Attending School
By daniel mollenkamp, more from edsurge.

How District Leaders Make Edtech Purchasing Decisions
By caitlin mclemore.

Are We There Yet? Skills-Based Technologies, Hiring and Advancement

Teaching and Learning
When students are absent, do their relationships with teachers suffer.

Textbooks and Course Materials
Federal rule change may undermine ‘inclusive access’ textbook models, by rebecca koenig.
Journalism that ignites your curiosity about education.
EdSurge is an editorially independent project of and
- Product Index
- Write for us
- Advertising
FOLLOW EDSURGE
© 2024 All Rights Reserved
Homework could have an impact on kids’ health. Should schools ban it?
Professor of Education, Penn State
Disclosure statement
Gerald K. LeTendre has received funding from the National Science Foundation and the Spencer Foundation.
Penn State provides funding as a founding partner of The Conversation US.
View all partners

Reformers in the Progressive Era (from the 1890s to 1920s) depicted homework as a “sin” that deprived children of their playtime . Many critics voice similar concerns today.
Yet there are many parents who feel that from early on, children need to do homework if they are to succeed in an increasingly competitive academic culture. School administrators and policy makers have also weighed in, proposing various policies on homework .
So, does homework help or hinder kids?
For the last 10 years, my colleagues and I have been investigating international patterns in homework using databases like the Trends in Mathematics and Science Study (TIMSS) . If we step back from the heated debates about homework and look at how homework is used around the world, we find the highest homework loads are associated with countries that have lower incomes and higher social inequality.
Does homework result in academic success?
Let’s first look at the global trends on homework.
Undoubtedly, homework is a global phenomenon ; students from all 59 countries that participated in the 2007 Trends in Math and Science Study (TIMSS) reported getting homework. Worldwide, only less than 7% of fourth graders said they did no homework.
TIMSS is one of the few data sets that allow us to compare many nations on how much homework is given (and done). And the data show extreme variation.
For example, in some nations, like Algeria, Kuwait and Morocco, more than one in five fourth graders reported high levels of homework. In Japan, less than 3% of students indicated they did more than four hours of homework on a normal school night.
TIMSS data can also help to dispel some common stereotypes. For instance, in East Asia, Hong Kong, Taiwan and Japan – countries that had the top rankings on TIMSS average math achievement – reported rates of heavy homework that were below the international mean.
In the Netherlands, nearly one out of five fourth graders reported doing no homework on an average school night, even though Dutch fourth graders put their country in the top 10 in terms of average math scores in 2007.
Going by TIMSS data, the US is neither “ A Nation at Rest” as some have claimed, nor a nation straining under excessive homework load . Fourth and eighth grade US students fall in the middle of the 59 countries in the TIMSS data set, although only 12% of US fourth graders reported high math homework loads compared to an international average of 21%.
So, is homework related to high academic success?
At a national level, the answer is clearly no. Worldwide, homework is not associated with high national levels of academic achievement .
But, the TIMSS can’t be used to determine if homework is actually helping or hurting academic performance overall , it can help us see how much homework students are doing, and what conditions are associated with higher national levels of homework.
We have typically found that the highest homework loads are associated with countries that have lower incomes and higher levels of social inequality – not hallmarks that most countries would want to emulate.
Impact of homework on kids
TIMSS data also show us how even elementary school kids are being burdened with large amounts of homework.
Almost 10% of fourth graders worldwide (one in 10 children) reported spending multiple hours on homework each night. Globally, one in five fourth graders report 30 minutes or more of homework in math three to four times a week.
These reports of large homework loads should worry parents, teachers and policymakers alike.
Empirical studies have linked excessive homework to sleep disruption , indicating a negative relationship between the amount of homework, perceived stress and physical health.

What constitutes excessive amounts of homework varies by age, and may also be affected by cultural or family expectations. Young adolescents in middle school, or teenagers in high school, can study for longer duration than elementary school children.
But for elementary school students, even 30 minutes of homework a night, if combined with other sources of academic stress, can have a negative impact . Researchers in China have linked homework of two or more hours per night with sleep disruption .
Even though some cultures may normalize long periods of studying for elementary age children, there is no evidence to support that this level of homework has clear academic benefits . Also, when parents and children conflict over homework, and strong negative emotions are created, homework can actually have a negative association with academic achievement.
Should there be “no homework” policies?
Administrators and policymakers have not been reluctant to wade into the debates on homework and to formulate policies . France’s president, Francois Hollande, even proposed that homework be banned because it may have inegaliatarian effects.
However, “zero-tolerance” homework policies for schools, or nations, are likely to create as many problems as they solve because of the wide variation of homework effects. Contrary to what Hollande said, research suggests that homework is not a likely source of social class differences in academic achievement .
Homework, in fact, is an important component of education for students in the middle and upper grades of schooling.
Policymakers and researchers should look more closely at the connection between poverty, inequality and higher levels of homework. Rather than seeing homework as a “solution,” policymakers should question what facets of their educational system might impel students, teachers and parents to increase homework loads.
At the classroom level, in setting homework, teachers need to communicate with their peers and with parents to assure that the homework assigned overall for a grade is not burdensome, and that it is indeed having a positive effect.
Perhaps, teachers can opt for a more individualized approach to homework. If teachers are careful in selecting their assignments – weighing the student’s age, family situation and need for skill development – then homework can be tailored in ways that improve the chance of maximum positive impact for any given student.
I strongly suspect that when teachers face conditions such as pressure to meet arbitrary achievement goals, lack of planning time or little autonomy over curriculum, homework becomes an easy option to make up what could not be covered in class.
Whatever the reason, the fact is a significant percentage of elementary school children around the world are struggling with large homework loads. That alone could have long-term negative consequences for their academic success.
- Trends in Mathematics and Science Study (TIMSS)
- Elementary school
- Academic success

Campus Life Services Officer

Apply for State Library of Queensland's next round of research opportunities

Associate Professor, Psychology

Professor and Head of School, School of Communication and Arts

Management Information Systems & Analytics – Limited Term Contract

- Mental Health , Research
- Written by Cara Goodwin, Ph.D.
Homework: The Good and The Bad

Homework. A single word that for many brings up memories of childhood stress. Now that you’re a parent, you may be reminded of that feeling every time your child spills their backpack across the table. You also may be questioning how much homework is too much and wondering how you can best help your child with their schoolwork.
Here, Dr. Cara Goodwin of Parenting Translator explains what the research actually says about homework. She outlines specific ways parents can support their kids to maximize the academic benefits and develop lifelong skills in time management and persistence.
In recent years, many parents and educators have raised concerns about homework. Specifically, they have questioned how much it enhances learning and if its benefits outweigh potential costs, such as stress to the family.
So, what does the research say?
Academic benefits vs risks of homework
One of the most important questions when it comes to homework is whether it actually helps kids understand the content better. So does it? Research finds that homework is associated with higher scores on academic standardized tests for middle and high school students, but not for elementary school students (1, 2).
In other words, homework seems to have little impact on learning in elementary school students.
Additionally, a 2012 study found that while homework is related to higher standardized test scores for high schoolers, it is not related to higher grades.
Not surprisingly, homework is more likely to be associated with improved academic performance when students and teachers find the homework to be meaningful or relevant, according to several studies (1, 3, 4). Students tend to find homework to be most engaging when it involves solving real-world problems (5).
The impact of homework may also depend on socioeconomic status. Students from higher income families show improved academic skills with more homework and gain more knowledge from homework, according to research. On the other hand, the academic performance of more disadvantaged children seems to be unaffected by homework (6, 7). This may be because homework provides additional stress for disadvantaged children. They are less likely to get help from their parents on homework and more likely to be punished by teachers for not completing it (8).
Non-academic benefits vs risks of homework
Academic outcomes are only part of the picture. It is important to look at how homework affects kids in ways other than grades and test scores.
Homework appears to have benefits beyond improving academic skills, particularly for younger students. These benefits include building responsibility, time management skills, and persistence (1, 9, 10). In addition, homework may also increase parents’ involvement in their children’s schooling (11, 12, 13, 14).
Yet, studies show that too much homework has drawbacks. It can reduce children’s opportunities for free play, which is essential for the development of language, cognitive, self-regulation, and social-emotional skills (15). It may also interfere with physical activity, and too much homework is associated with an increased risk for being overweight (16, 17).
In addition to homework reducing opportunities for play, it also leads to increased conflicts and stress for families. For example, research finds that children with more hours of homework experience more academic stress, physical health problems, and lack of balance in their lives (18).
Clearly, more is not better when it comes to homework.
What is the “right” amount of homework?
Recent reports indicate that elementary school students are assigned three times the recommended amount of homework. Even kindergarten students report an average of 25 minutes of homework per day (19).
Additionally, the National Assessment of Educational Progress (NAEP) found that homework has been increasing in recent years for younger students. Specifically, 35% of 9-year-olds reported that they did not do homework the previous night in 1984 versus 22% of 9-years-old in 2012. However, homework levels have stayed relatively stable for 13- and 17-year-olds during this same time period.
Research suggests that homework should not exceed 1.5 to 2.5 hours per night for high school students and no more than 1 hour per night for middle school students (1). Homework for elementary school students should be minimal and assigned with the aim of building self-regulation and independent work skills. A common rule , supported by both the National Education Association (NEA) and National Parent Teacher Association (PTA), is 10-minutes of homework per grade in elementary school. Any more than this and homework may no longer have a positive impact. Importantly, the NEA and the National PTA do not endorse homework for kindergarteners.
How can parents best help with homework?
Most parents feel that they are expected to be involved in their children’s homework (20). Yet, it is often unclear exactly how to be involved in a way that helps your child to successfully complete the assignment without taking over entirely. Most studies find that parental help is important but that it matters more HOW the parent is helping rather than how OFTEN the parent is helping (21).
While this can all feel very overwhelming for parents, there are some simple guidelines you can follow to ease the homework burden and best support your child’s learning.
1. Help only when needed.
Parents should focus on providing general monitoring, guidance and encouragement. Allow children to generate answers on their own and complete their homework as independently as possible . This is important because research shows that allowing children more independence in completing homework benefits their academic skills (22, 23). In addition, too much parent involvement and being controlling with homework is associated with worse academic performance (21, 24, 25).
What does this look like?
- Be present when your child is completing homework to help them to understand the directions.
- Be available to answer simple questions and to provide praise for their effort and hard work.
- Only provide help when your child asks for it and step away whenever possible.
2. Have structure and routines.
Help your child create structure and to develop some routines. This helps children become more independent in completing their homework. Research finds that providing this type of structure and responsiveness is related to improved academic skills (25).
This structure may include:
- A regular time and place for homework that is free from distractions.
- Have all of the materials they need within arm’s reach.
- Teach and encourage kids to create a checklist for their homework tasks each day.
Parents can also help their children to find ways to stay motivated. For example, developing their own reward system or creating a homework schedule with breaks for fun activities.
3. Set specific rules around homework.
Research finds that parents setting rules around homework is related to higher academic performance (26). For example, parents may require that children finish homework before screen time or may require children to stop doing homework and go to sleep at a certain hour.
4. Emphasize learning over outcome.
Encourage your child to persist in challenging assignments and frame difficult assignments as opportunities to grow. Research finds that this attitude is associated with student success (20). Research also indicates that more challenging homework is associated with enhanced school performance (27).
Additionally, help your child to view homework as an opportunity to learn and improve skills. Parents who view homework as a learning opportunity rather than something that they must get “right” or complete successfully to obtain a higher grade are more likely to have children with the same attitudes (28).
5. Stay calm and positive.
Yes, we know this is easier said than done, but it does have a big impact on how kids persevere when things get hard! Research shows that mothers showing positive emotions while helping with homework may improve children’s motivation in homework (29)
6. Praise hard work and effort.
Praise focused on effort is likely to increase motivation (30). In addition, research finds that putting more effort into homework may be associated with enhanced development of conscientiousness in children (31).
7. Communicate with your child’s teacher.
Let your child’s teacher know about any problems your child has with homework and the teachers’ learning goals. Research finds that open communication about homework is associated with improved school performance (32).

In summary, research finds that homework provides some academic benefit for middle- and high-school students but is less beneficial for elementary school students. As a parent, how you are involved in your child’s homework really matters. By following these evidence-based tips, you can help your child to maximize the benefits of homework and make the process less painful for all involved!
For more resources, take a look at our recent posts on natural and logical consequences and simple ways to decrease challenging behaviors .
- Cooper, H., Robinson, J. C., & Patall, E. A. (2006). Does homework improve academic achievement? A synthesis of research, 1987–2003. Review of educational research , 76 (1), 1-62.
- Muhlenbruck, L., Cooper, H., Nye, B., & Lindsay, J. J. (1999). Homework and achievement: Explaining the different strengths of relation at the elementary and secondary school levels. Social Psychology of Education , 3 (4), 295-317.
- Marzano, R. J., & Pickering, D. J. (2007). Special topic: The case for and against homework. Educational leadership , 64 (6), 74-79.
- Trautwein, U., Lüdtke, O., Schnyder, I., & Niggli, A. (2006). Predicting homework effort: support for a domain-specific, multilevel homework model. Journal of educational psychology , 98 (2), 438.
- Shernoff, D. J., Csikszentmihalyi, M., Schneider, B., & Shernoff, E. S. (2014). Student engagement in high school classrooms from the perspective of flow theory. In Applications of flow in human development and education (pp. 475-494). Springer, Dordrecht.
- Daw, J. (2012). Parental income and the fruits of labor: Variability in homework efficacy in secondary school. Research in social stratification and mobility , 30 (3), 246-264.
- Rønning, M. (2011). Who benefits from homework assignments?. Economics of Education Review , 30 (1), 55-64.
- Calarco, J. M. (2020). Avoiding us versus them: How schools’ dependence on privileged “Helicopter” parents influences enforcement of rules. American Sociological Review , 85 (2), 223-246.
- Corno, L., & Xu, J. (2004). Homework as the job of childhood. Theory into practice , 43 (3), 227-233.
- Göllner, R., Damian, R. I., Rose, N., Spengler, M., Trautwein, U., Nagengast, B., & Roberts, B. W. (2017). Is doing your homework associated with becoming more conscientious?. Journal of Research in Personality , 71 , 1-12.
- Balli, S. J., Demo, D. H., & Wedman, J. F. (1998). Family involvement with children’s homework: An intervention in the middle grades. Family relations , 149-157.
- Balli, S. J., Wedman, J. F., & Demo, D. H. (1997). Family involvement with middle-grades homework: Effects of differential prompting. The Journal of Experimental Education , 66 (1), 31-48.
- Epstein, J. L., & Dauber, S. L. (1991). School programs and teacher practices of parent involvement in inner-city elementary and middle schools. The elementary school journal , 91 (3), 289-305.
- Van Voorhis, F. L. (2003). Interactive homework in middle school: Effects on family involvement and science achievement. The Journal of Educational Research , 96 (6), 323-338.
- Yogman, M., Garner, A., Hutchinson, J., Hirsh-Pasek, K., Golinkoff, R. M., & Committee on Psychosocial Aspects of Child and Family Health. (2018). The power of play: A pediatric role in enhancing development in young children. Pediatrics , 142 (3).
- Godakanda, I., Abeysena, C., & Lokubalasooriya, A. (2018). Sedentary behavior during leisure time, physical activity and dietary habits as risk factors of overweight among school children aged 14–15 years: case control study. BMC research notes , 11 (1), 1-6.
- Hadianfard, A. M., Mozaffari-Khosravi, H., Karandish, M., & Azhdari, M. (2021). Physical activity and sedentary behaviors (screen time and homework) among overweight or obese adolescents: a cross-sectional observational study in Yazd, Iran. BMC pediatrics , 21 (1), 1-10.
- Galloway, M., Conner, J., & Pope, D. (2013). Nonacademic effects of homework in privileged, high-performing high schools. The journal of experimental education , 81 (4), 490-510.
- Pressman, R. M., Sugarman, D. B., Nemon, M. L., Desjarlais, J., Owens, J. A., & Schettini-Evans, A. (2015). Homework and family stress: With consideration of parents’ self confidence, educational level, and cultural background. The American Journal of Family Therapy , 43 (4), 297-313.
- Hoover-Dempsey, K. V., Battiato, A. C., Walker, J. M., Reed, R. P., DeJong, J. M., & Jones, K. P. (2001). Parental involvement in homework. Educational psychologist , 36 (3), 195-209.
- Moroni, S., Dumont, H., Trautwein, U., Niggli, A., & Baeriswyl, F. (2015). The need to distinguish between quantity and quality in research on parental involvement: The example of parental help with homework. The Journal of Educational Research , 108 (5), 417-431.
- Cooper, H., Lindsay, J. J., & Nye, B. (2000). Homework in the home: How student, family, and parenting-style differences relate to the homework process. Contemporary educational psychology , 25 (4), 464-487.
- Dumont, H., Trautwein, U., Lüdtke, O., Neumann, M., Niggli, A., & Schnyder, I. (2012). Does parental homework involvement mediate the relationship between family background and educational outcomes?. Contemporary Educational Psychology , 37 (1), 55-69.
- Barger, M. M., Kim, E. M., Kuncel, N. R., & Pomerantz, E. M. (2019). The relation between parents’ involvement in children’s schooling and children’s adjustment: A meta-analysis. Psychological bulletin , 145 (9), 855.
- Dumont, H., Trautwein, U., Nagy, G., & Nagengast, B. (2014). Quality of parental homework involvement: predictors and reciprocal relations with academic functioning in the reading domain. Journal of Educational Psychology , 106 (1), 144.
- Patall, E. A., Cooper, H., & Robinson, J. C. (2008). The effects of choice on intrinsic motivation and related outcomes: a meta-analysis of research findings. Psychological bulletin , 134 (2), 270.Dettmars et al., 2010
- Madjar, Shklar, & Moshe, 2016)
- Pomerantz, E. M., Grolnick, W. S., & Price, C. E. (2005). The Role of Parents in How Children Approach Achievement: A Dynamic Process Perspective.
- Haimovitz, K., Wormington, S. V., & Corpus, J. H. (2011). Dangerous mindsets: How beliefs about intelligence predict motivational change. Learning and Individual Differences , 21 (6), 747-752.Gollner et al., 2017
- Hill, N. E., & Tyson, D. F. (2009). Parental involvement in middle school: a meta-analytic assessment of the strategies that promote achievement. Developmental psychology , 45 (3), 740.
Share this post
11 Comments
Pingback: nfl|nfl highlights|nfl draft|nfl theme|nfl halftime show|nfl theme song|nfl draft 2023|nfl super bowl 2023|nfl 23|nfl 22|nfl halftime show 2022|nfl news|nfl live|nfl mock draft 2023|NFL player collapse|NFL live coverage|NFL Playoffs 2023|NFL game|NFL pred
Pingback: dutch driver license
Pingback: หวยออนไลน์ LSM99
Pingback: biracial silicone dolls
Pingback: เช่ารถตู้พร้อมคนขับ
Pingback: iTunes gift card
Pingback: fuckgirl
Pingback: go88 tài xỉu
Pingback: เสริมหน้าอก
Pingback: ชอบหีสวย.com
Pingback: gunpowder
Comments are closed.

As psychologists, we were passionate about evidence-based parenting even before having kids ourselves. Once we became parents, we were overwhelmed by the amount of parenting information available, some of which isn’t backed by research. This inspired the Helping Families Thrive mission: to bring parenting science to the real world.
search the site

Learn With Us
Psychologist created, parent tested workshops and mini-courses to help families thrive.
Our comprehensive Essentials course puts the power of the most studied parenting tools in the palm of your hand.
post categories

Improve Your Child’s Emotion Regulation Skills
A free, 15-page guide filled with practical strategies.

find us elsewhere
find your way around
discover courses
important links

Our 3-Step Strategy
Download our free guide to improve your child’s cooperation.
For educational purposes only. Not intended to diagnose or treat any condition, illness or disease.

When Is Homework Stressful? Its Effects on Students’ Mental Health

Are you wondering when is homework stressful? Well, homework is a vital constituent in keeping students attentive to the course covered in a class. By applying the lessons, students learned in class, they can gain a mastery of the material by reflecting on it in greater detail and applying what they learned through homework.
However, students get advantages from homework, as it improves soft skills like organisation and time management which are important after high school. However, the additional work usually causes anxiety for both the parents and the child. As their load of homework accumulates, some students may find themselves growing more and more bored.
Students may take assistance online and ask someone to do my online homework . As there are many platforms available for the students such as Chegg, Scholarly Help, and Quizlet offering academic services that can assist students in completing their homework on time.
Negative impact of homework
There are the following reasons why is homework stressful and leads to depression for students and affect their mental health. As they work hard on their assignments for alarmingly long periods, students’ mental health is repeatedly put at risk. Here are some serious arguments against too much homework.
No uniqueness
Homework should be intended to encourage children to express themselves more creatively. Teachers must assign kids intriguing assignments that highlight their uniqueness. similar to writing an essay on a topic they enjoy.
Moreover, the key is encouraging the child instead of criticizing him for writing a poor essay so that he can express himself more creatively.
Lack of sleep
One of the most prevalent adverse effects of schoolwork is lack of sleep. The average student only gets about 5 hours of sleep per night since they stay up late to complete their homework, even though the body needs at least 7 hours of sleep every day. Lack of sleep has an impact on both mental and physical health.
No pleasure
Students learn more effectively while they are having fun. They typically learn things more quickly when their minds are not clouded by fear. However, the fear factor that most teachers introduce into homework causes kids to turn to unethical means of completing their assignments.
Excessive homework
The lack of coordination between teachers in the existing educational system is a concern. As a result, teachers frequently end up assigning children far more work than they can handle. In such circumstances, children turn to cheat on their schoolwork by either copying their friends’ work or using online resources that assist with homework.
Anxiety level
Homework stress can increase anxiety levels and that could hurt the blood pressure norms in young people . Do you know? Around 3.5% of young people in the USA have high blood pressure. So why is homework stressful for children when homework is meant to be enjoyable and something they look forward to doing? It is simple to reject this claim by asserting that schoolwork is never enjoyable, yet with some careful consideration and preparation, homework may become pleasurable.
No time for personal matters
Students that have an excessive amount of homework miss out on personal time. They can’t get enough enjoyment. There is little time left over for hobbies, interpersonal interaction with colleagues, and other activities.
However, many students dislike doing their assignments since they don’t have enough time. As they grow to detest it, they can stop learning. In any case, it has a significant negative impact on their mental health.
Children are no different than everyone else in need of a break. Weekends with no homework should be considered by schools so that kids have time to unwind and prepare for the coming week. Without a break, doing homework all week long might be stressful.
How do parents help kids with homework?
Encouraging children’s well-being and health begins with parents being involved in their children’s lives. By taking part in their homework routine, you can see any issues your child may be having and offer them the necessary support.

Set up a routine
Your student will develop and maintain good study habits if you have a clear and organized homework regimen. If there is still a lot of schoolwork to finish, try putting a time limit. Students must obtain regular, good sleep every single night.
Observe carefully
The student is ultimately responsible for their homework. Because of this, parents should only focus on ensuring that their children are on track with their assignments and leave it to the teacher to determine what skills the students have and have not learned in class.
Listen to your child
One of the nicest things a parent can do for their kids is to ask open-ended questions and listen to their responses. Many kids are reluctant to acknowledge they are struggling with their homework because they fear being labelled as failures or lazy if they do.
However, every parent wants their child to succeed to the best of their ability, but it’s crucial to be prepared to ease the pressure if your child starts to show signs of being overburdened with homework.
Talk to your teachers
Also, make sure to contact the teacher with any problems regarding your homework by phone or email. Additionally, it demonstrates to your student that you and their teacher are working together to further their education.
Homework with friends
If you are still thinking is homework stressful then It’s better to do homework with buddies because it gives them these advantages. Their stress is reduced by collaborating, interacting, and sharing with peers.
Additionally, students are more relaxed when they work on homework with pals. It makes even having too much homework manageable by ensuring they receive the support they require when working on the assignment. Additionally, it improves their communication abilities.
However, doing homework with friends guarantees that one learns how to communicate well and express themselves.
Review homework plan
Create a schedule for finishing schoolwork on time with your child. Every few weeks, review the strategy and make any necessary adjustments. Gratefully, more schools are making an effort to control the quantity of homework assigned to children to lessen the stress this produces.
Bottom line
Finally, be aware that homework-related stress is fairly prevalent and is likely to occasionally affect you or your student. Sometimes all you or your kid needs to calm down and get back on track is a brief moment of comfort. So if you are a student and wondering if is homework stressful then you must go through this blog.
While homework is a crucial component of a student’s education, when kids are overwhelmed by the amount of work they have to perform, the advantages of homework can be lost and grades can suffer. Finding a balance that ensures students understand the material covered in class without becoming overburdened is therefore essential.
Zuella Montemayor did her degree in psychology at the University of Toronto. She is interested in mental health, wellness, and lifestyle.

Psychreg is a digital media company and not a clinical company. Our content does not constitute a medical or psychological consultation. See a certified medical or mental health professional for diagnosis.
- Privacy Policy
© Copyright 2014–2034 Psychreg Ltd
- PSYCHREG JOURNAL
- MEET OUR WRITERS
- MEET THE TEAM
Request More Info
Fill out the form below and a member of our team will reach out right away!
" * " indicates required fields
Is Homework Necessary? Education Inequity and Its Impact on Students

The Problem with Homework: It Highlights Inequalities
How much homework is too much homework, when does homework actually help, negative effects of homework for students, how teachers can help.
Schools are getting rid of homework from Essex, Mass., to Los Angeles, Calif. Although the no-homework trend may sound alarming, especially to parents dreaming of their child’s acceptance to Harvard, Stanford or Yale, there is mounting evidence that eliminating homework in grade school may actually have great benefits , especially with regard to educational equity.
In fact, while the push to eliminate homework may come as a surprise to many adults, the debate is not new . Parents and educators have been talking about this subject for the last century, so that the educational pendulum continues to swing back and forth between the need for homework and the need to eliminate homework.
One of the most pressing talking points around homework is how it disproportionately affects students from less affluent families. The American Psychological Association (APA) explained:
“Kids from wealthier homes are more likely to have resources such as computers, internet connections, dedicated areas to do schoolwork and parents who tend to be more educated and more available to help them with tricky assignments. Kids from disadvantaged homes are more likely to work at afterschool jobs, or to be home without supervision in the evenings while their parents work multiple jobs.”
[RELATED] How to Advance Your Career: A Guide for Educators >>
While students growing up in more affluent areas are likely playing sports, participating in other recreational activities after school, or receiving additional tutoring, children in disadvantaged areas are more likely headed to work after school, taking care of siblings while their parents work or dealing with an unstable home life. Adding homework into the mix is one more thing to deal with — and if the student is struggling, the task of completing homework can be too much to consider at the end of an already long school day.
While all students may groan at the mention of homework, it may be more than just a nuisance for poor and disadvantaged children, instead becoming another burden to carry and contend with.
Beyond the logistical issues, homework can negatively impact physical health and stress — and once again this may be a more significant problem among economically disadvantaged youth who typically already have a higher stress level than peers from more financially stable families .
Yet, today, it is not just the disadvantaged who suffer from the stressors that homework inflicts. A 2014 CNN article, “Is Homework Making Your Child Sick?” , covered the issue of extreme pressure placed on children of the affluent. The article looked at the results of a study surveying more than 4,300 students from 10 high-performing public and private high schools in upper-middle-class California communities.
“Their findings were troubling: Research showed that excessive homework is associated with high stress levels, physical health problems and lack of balance in children’s lives; 56% of the students in the study cited homework as a primary stressor in their lives,” according to the CNN story. “That children growing up in poverty are at-risk for a number of ailments is both intuitive and well-supported by research. More difficult to believe is the growing consensus that children on the other end of the spectrum, children raised in affluence, may also be at risk.”
When it comes to health and stress it is clear that excessive homework, for children at both ends of the spectrum, can be damaging. Which begs the question, how much homework is too much?
The National Education Association and the National Parent Teacher Association recommend that students spend 10 minutes per grade level per night on homework . That means that first graders should spend 10 minutes on homework, second graders 20 minutes and so on. But a study published by The American Journal of Family Therapy found that students are getting much more than that.
While 10 minutes per day doesn’t sound like much, that quickly adds up to an hour per night by sixth grade. The National Center for Education Statistics found that high school students get an average of 6.8 hours of homework per week, a figure that is much too high according to the Organization for Economic Cooperation and Development (OECD). It is also to be noted that this figure does not take into consideration the needs of underprivileged student populations.
In a study conducted by the OECD it was found that “after around four hours of homework per week, the additional time invested in homework has a negligible impact on performance .” That means that by asking our children to put in an hour or more per day of dedicated homework time, we are not only not helping them, but — according to the aforementioned studies — we are hurting them, both physically and emotionally.
What’s more is that homework is, as the name implies, to be completed at home, after a full day of learning that is typically six to seven hours long with breaks and lunch included. However, a study by the APA on how people develop expertise found that elite musicians, scientists and athletes do their most productive work for about only four hours per day. Similarly, companies like Tower Paddle Boards are experimenting with a five-hour workday, under the assumption that people are not able to be truly productive for much longer than that. CEO Stephan Aarstol told CNBC that he believes most Americans only get about two to three hours of work done in an eight-hour day.
In the scope of world history, homework is a fairly new construct in the U.S. Students of all ages have been receiving work to complete at home for centuries, but it was educational reformer Horace Mann who first brought the concept to America from Prussia.
Since then, homework’s popularity has ebbed and flowed in the court of public opinion. In the 1930s, it was considered child labor (as, ironically, it compromised children’s ability to do chores at home). Then, in the 1950s, implementing mandatory homework was hailed as a way to ensure America’s youth were always one step ahead of Soviet children during the Cold War. Homework was formally mandated as a tool for boosting educational quality in 1986 by the U.S. Department of Education, and has remained in common practice ever since.
School work assigned and completed outside of school hours is not without its benefits. Numerous studies have shown that regular homework has a hand in improving student performance and connecting students to their learning. When reviewing these studies, take them with a grain of salt; there are strong arguments for both sides, and only you will know which solution is best for your students or school.
Homework improves student achievement.
- Source: The High School Journal, “ When is Homework Worth the Time?: Evaluating the Association between Homework and Achievement in High School Science and Math ,” 2012.
- Source: IZA.org, “ Does High School Homework Increase Academic Achievement? ,” 2014. **Note: Study sample comprised only high school boys.
Homework helps reinforce classroom learning.
- Source: “ Debunk This: People Remember 10 Percent of What They Read ,” 2015.
Homework helps students develop good study habits and life skills.
- Sources: The Repository @ St. Cloud State, “ Types of Homework and Their Effect on Student Achievement ,” 2017; Journal of Advanced Academics, “ Developing Self-Regulation Skills: The Important Role of Homework ,” 2011.
- Source: Journal of Advanced Academics, “ Developing Self-Regulation Skills: The Important Role of Homework ,” 2011.
Homework allows parents to be involved with their children’s learning.
- Parents can see what their children are learning and working on in school every day.
- Parents can participate in their children’s learning by guiding them through homework assignments and reinforcing positive study and research habits.
- Homework observation and participation can help parents understand their children’s academic strengths and weaknesses, and even identify possible learning difficulties.
- Source: Phys.org, “ Sociologist Upends Notions about Parental Help with Homework ,” 2018.
While some amount of homework may help students connect to their learning and enhance their in-class performance, too much homework can have damaging effects.
Students with too much homework have elevated stress levels.
- Source: USA Today, “ Is It Time to Get Rid of Homework? Mental Health Experts Weigh In ,” 2021.
- Source: Stanford University, “ Stanford Research Shows Pitfalls of Homework ,” 2014.
Students with too much homework may be tempted to cheat.
- Source: The Chronicle of Higher Education, “ High-Tech Cheating Abounds, and Professors Bear Some Blame ,” 2010.
- Source: The American Journal of Family Therapy, “ Homework and Family Stress: With Consideration of Parents’ Self Confidence, Educational Level, and Cultural Background ,” 2015.
Homework highlights digital inequity.
- Sources: NEAToday.org, “ The Homework Gap: The ‘Cruelest Part of the Digital Divide’ ,” 2016; CNET.com, “ The Digital Divide Has Left Millions of School Kids Behind ,” 2021.
- Source: Investopedia, “ Digital Divide ,” 2022; International Journal of Education and Social Science, “ Getting the Homework Done: Social Class and Parents’ Relationship to Homework ,” 2015.
- Source: World Economic Forum, “ COVID-19 exposed the digital divide. Here’s how we can close it ,” 2021.
Homework does not help younger students.
- Source: Review of Educational Research, “ Does Homework Improve Academic Achievement? A Synthesis of Researcher, 1987-2003 ,” 2006.
To help students find the right balance and succeed, teachers and educators must start the homework conversation, both internally at their school and with parents. But in order to successfully advocate on behalf of students, teachers must be well educated on the subject, fully understanding the research and the outcomes that can be achieved by eliminating or reducing the homework burden. There is a plethora of research and writing on the subject for those interested in self-study.
For teachers looking for a more in-depth approach or for educators with a keen interest in educational equity, formal education may be the best route. If this latter option sounds appealing, there are now many reputable schools offering online master of education degree programs to help educators balance the demands of work and family life while furthering their education in the quest to help others.
YOU’RE INVITED! Watch Free Webinar on USD’s Online MEd Program >>
Be Sure To Share This Article
- Share on Twitter
- Share on Facebook
- Share on LinkedIn
Top 11 Reasons to get Your Master of Education Degree
Free 22-page Book

- Master of Education
Related Posts


Homework can be bad for your mental health. Should we get rid of it?
- Teachers are taking to social media apps like TikTok to take a stand against the idea
- Heavy workloads can be extra stressful for students, especially during the Covid-19 pandemic
Latest Articles
Brazilian flood reveals ‘well-preserved’ dinosaur fossil dating back 200 million years, face off: should hong kong universities charge students more for higher-costing courses, your voice: responsible social media use, mental health issues and harmful effects of helicopter parenting, cambridge art exhibition goes down memory lane to the 1924 paris olympics, ‘twisters’ is an enjoyable holiday hit that might be better than the first film, mentorship programme helps ethnically diverse hongkongers launch their businesses.

It’s no secret that homework can be a pain. And as students grapple with an ongoing pandemic that has had a wide-range of mental health impacts, is it time schools start listening to their pleas about their workloads?
Some teachers are turning to social media to take a stand against homework.
Tiktok user @misguided.teacher says he doesn’t assign it because the “whole premise of homework is flawed.”
Do students need homework to learn?
For starters, he says he can’t grade work on “even playing fields” when students’ home environments can be vastly different.
“Even students who go home to a peaceful house, do they really want to spend their time on busy work? Because typically that’s what a lot of homework is, it’s busy work,” he says in the video that has garnered 1.6 million likes. “You only get one year to be 7, you only got one year to be 10, you only get one year to be 16, 18.”
Mental health experts agree heavy work loads have the potential do more harm than good for students, especially when taking into account the impacts of the pandemic. But they also say the answer may not be to eliminate homework altogether.
Chinese schoolgirl uses robot to do her homework
Emmy Kang, mental health counselor at Humantold, says studies have shown heavy workloads can be “detrimental” for students and cause a “big impact on their mental, physical and emotional health.”
“More than half of students say that homework is their primary source of stress, and we know what stress can do on our bodies,” she says, adding that staying up late to finish assignments also leads to disrupted sleep and exhaustion.
Cynthia Catchings, a licensed clinical social worker and therapist at Talkspace, says heavy workloads can also cause serious mental health problems in the long run, like anxiety and depression.
And for all the distress homework causes, it’s not as useful as many may think, says Dr. Nicholas Kardaras, a psychologist and CEO of Omega Recovery treatment center.
“The research shows that there’s really limited benefit of homework for elementary age students, that really the school work should be contained in the classroom,” he says.
For older students, Kang says homework benefits plateau at about two hours per night.
Should Hong Kong introduce a zero-homework policy?
“Most students, especially at these high-achieving schools, they’re doing a minimum of three hours, and it’s taking away time from their friends from their families, their extracurricular activities. And these are all very important things for a person’s mental and emotional health.”
Catchings, who taught Primary Three to Secondary 6 for 12 years, says she’s seen the positive effects of a no homework policy while working with students abroad.
“Not having homework was something that I always admired from French students (and) French schools, because that helped students really have time off and disconnect from school,” she says.
The answer may not be to eliminate homework completely, but to be more mindful of the type of work students go home with, suggests Kang, who was a secondary school teacher for 10 years.
“I don’t think (we) should scrap homework, I think we should scrap meaningless, purposeless, busy work-type homework. That’s something that needs to be scrapped entirely,” she says, encouraging teachers to be thoughtful and consider the amount of time it would take for students to complete assignments.
- entertainment
- journalism resources
- latest reports
- learning resources
- college & uni life
- career advice
trending topics
Can Excessive Homework Cause Depression? – 17 Reasons

Does Homework Cause Depression?
You or a loved one may currently be struggling with excessive amounts of homework and are wondering if can homework cause depression. The short answer is that depression can absolutely lead to depression for a variety of reasons. Identifying the cause of your homework-related depression below is the first step toward bettering your mental health. Excessive homework, lack of social support, and perfectionism are just a few of the reasons homework can lead to depression.
Reasons Why Homework Might Cause Depression:
1) multiple hours of homework.
Starting in high school, or sometimes even middle school, students begin to slowly receive more and more hours of homework. There are various reasons why multiple hours of homework per week might lead students to depression, with stress and procrastination being at the top. Certain subjects, such as mathematics or science-related classes (i.e. chemistry) might also be challenging for some students to comprehend, leading them to feel drowned in stress and procrastination. Many hours of homework on a subject that one does not understand may start as stress and procrastination, but across multiple weeks or months may gradually turn into strong feelings of inadequacy and ultimately depression.
2) Extracurricular Activities May Interfere
Only some people in high school are distinguished academics capable of effortlessly finishing hours of homework each week. Other students are naturally gifted in different areas, such as athletics, arts (such as music or drawing), or even gaming! However, these activities can also get in the way of valuable time each student needs to complete homework and be successful in school. If the student prioritizes extracurricular activities significantly more than homework, this certainly can have negative mental health consequences. Ultimately, a school/leisure balance is key when it comes to avoiding student depression and maintaining overall happiness and success!
3) Excessive Homework From Classes or Teachers
Certain subjects or teachers might also be prone to assign excessive homework that might initially be daunting. For example, mathematics, chemistry, and physics are examples of classes that high school students find both challenging and overwhelming. If these classes are not already difficult enough, a bad professor might add excessive homework that is clearly not necessary for the student’s growth. The combination of excessive homework and poor teachers can easily lead students to mild depression over time.
4) Prior Struggles With Mental Health Issues
Mental health issues in the United States and across the world are higher now than at any point in much of human history. This means that many students already have mild to severe forms of depression, anxiety, PTSD, and other mental health issues. When students receive homework that they feel incapable of completing with little to no support outside school, this may only worsen their mental health. Unfortunately, depression is only one of many mental health issues students may face when confronted with challenging homework.
5) The Sleep Deprivation Cycle
Many students, especially in high school and college, naturally prefer to stay up late and wake up late. These individuals are commonly referred to as “night owls” since they mostly thrive at night. Being a “night owl” is not an inherent problem until the student’s sleep begins to fall below the recommended range of 7-9 hours per night. Sleep deprivation is defined as “a state caused by inadequate quality or quantity of sleep.”
Although side effects of sleep deprivation depend on a few factors, depression is one of the most common. Students might feel stressed during the day and procrastinate until night when they make the logical decision to stay up and finally complete the homework for the following day. This is the day-to-day reality for many students since they have yet to break the sleep deprivation-depression cycle.
6) Concern With Getting Good Grades
Although getting good grades is applauded by almost everyone ranging from students and parents to teachers and principals, some students take it to the extreme. Perfectionism is the need to appear perfect, which might be reflected by extremely good grades or high GPAs (that are often unrealistic or unnecessary). Most colleges do not require students to have anywhere near perfect good grades or GPAs, meaning students should set realistic goals while still making it possible to achieve long-term goals. Setting realistic goals for good grades can also lead students to minimize stress, depression, and other negative effects that come with perfectionism.
7) Physical Health Problems
Some students might also suffer from physical health problems that are either genetic (such as Type I Diabetes) or behavioral health problems (such as obesity). In the case of a young student with obesity, his or her most difficult class might be the physical exercise (PE) class. This student might look at other students performing the daily physical activity for class and feel a sense of inadequacy that can grow over time and lead to depression, especially if not properly addressed or guided.
There is also a slew of other physical health problems that might contribute to a student’s struggle and depression when in school. These health problems might also make it difficult for students to complete homework when away from school, due to a variety of factors.
8) Weight Loss Problems (Homework Cause Depression)
Weight loss may occur when students are overly focused on school and have little to no time to eat a highly nutritious meal. Since food gives energy to the brain and is responsible for many vital functions of the human body, it is no wonder why depression might arise out of weight loss. In addition, many students, particularly young female students, go through a time that challenges their self-image. Other students and social media might pressure these students into conforming to non-realistic beauty standards via weight loss.
9) Young Adults and Lack of Balance
High schoolers and college students are still very young in the grand scheme of life, with many lacking the crucial ability to balance school and homework with other parts of their lives. Especially with the amount of homework some classes assign, students might not balance enough time that is necessary to complete the assigned work. Students might spend much time procrastinating homework and doing fun, but less productive activities, such as playing sports or video games. Mental health counselors (either at school or in a clinical setting) can be extremely effective at helping students manage school work and ultimately avoid depression.
10) Homework and Test Scores
High schoolers and older students must find enough time to complete homework and study to get optimal test scores. Although this does vary from class to class, most individuals will have at least a few classes where balancing homework and testing is critical for success. Test anxiety is a big factor that might lead students to heavily prioritize studying for an exam instead of completing homework. If students are overly concerned with test scores and neglect to complete assigned homework, depressive symptoms may occur.
11) The Importance of Time Management
As mentioned earlier, young children or young people, in general, might often struggle to effectively complete a lot of homework. Building daily habits around homework completion for just 30 minutes per day can add up to make a massive difference (that is 4.5 hours by the end of the school week!). Not only does effective time management make it easier to complete homework, but it also removes much of the stress, procrastination, and even depression that might come as a result of little to no time management.
For younger children or even high schoolers, an adult role model can significantly help develop these necessary habits sooner rather than later.
12) Prestigious Schools Like Stanford University
High schoolers looking to get accepted into prestigious colleges like Stanford University, or college students already in prestigious schools likely have higher chances to suffer from homework-related depression. High schoolers that are accepted into Stanford University have an average GPA of 3.95 out of 4.00.
This means students trying to get into ivy league schools hold themselves to an extreme standard. This extreme standard will inevitably cause a lack of sleep, depression, and a variety of other negative effects. This does not mean that it is impossible to successfully be accepted into Stanford University, it just means it will be very challenging.
13) Teachers Assign Busy Work
Sometimes teachers (mostly in high school) assign work that is repetitive, not super challenging, and time-consuming to complete. These types of assignments are generally referred to as “busy work,” and can be the bane of some high schoolers’ existence. The problem with busy work is that students begin to focus on the completion of the homework due to the sheer amount of time they know it will take to complete it. This takes away from the overall learning experience of the student and will lead many high school students to procrastinate. Procrastination can lead to piled-up homework and can have a negative impact on the student’s depression levels. Ultimately, teachers that assign busy homework cause depression.
14) Family Stress at Home (Homework Cause Depression)
Sometimes the cause of depression is much deeper than meets the eye, with homework simply exacerbating these untouched issues. One deeper issue revolves around family members and the lack of much-needed social support from parents, siblings, and other family members in the household. These family members might simply be unwilling to provide homework support to young adults, or the issue might be as bad as mental or physical abuse. If you know someone that is being abused, please seek help immediately to help them in the long run. It is clear that these issues could easily lead one to depression.
15) Lack of Friendships and Social Life
Being isolated at school and/or at home might be one of the risk factors for developing depression from homework. Friendships can be mutually beneficial when completing tasks such as homework since students are able to check each others’ work and reduce the overall stress of heavy workloads. Students that always seem to be alone or are even bullied might be at an increased risk of serious mental health problems. It is true that some young people and older students work best alone, but this is definitely a warning sign to keep an eye out for if you are a parent.
Putting isolated students into a club or sport they have an initial interest in might be a fantastic way to help them create valuable bonds with those around them and prevent depression!
16) Social Media and Student Well-Being
Social media is something that has had clear negative effects on the mental health of many age groups in the United States but also across the world. Social media often promotes the action of comparing one’s self to others, which might be academic success in this case. Individuals that are constantly watching other students succeed online may feel like they are the only one that does not understand the course material.
The amount of time spent on social media can also often take away from time that high schoolers could be spent completing homework and other important things. Ultimately, social media is best, like many things, when consumed in moderation and is not used to negatively compare oneself with others.
17) Stomach Problems Such as Celiac Disease
Stomach problems include, but are not limited to celiac disease, inflammatory bowel disease (IBD), lactose intolerance, and constipation or gas. Most of these stomach problems have nausea and even vomiting as some of their primary negative effects. Attempting to do homework or even come to school when having severe nausea is challenging, to say the least. Students with these issues will often have less time as a result, and may even feel as though homework cause depression.
From the list above, there are many clear reasons why excessive homework assignments might lead a student of any age to depression. If you or someone you know struggles with severe depression, please seek professional help. Although there are many ways homework can cause depression, we are strong and capable of overcoming the depression and still achieving success. Ultimately, social support from family and friends, academic guidance, and a consistent homework routine are just a few of the things that might help reduce depression caused by homework.
2 thoughts on “Can Excessive Homework Cause Depression? – 17 Reasons”
Wowwww!! This is extremely insightful ❤️🔥Can’t wait for your next post !
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

THE ALGONQUIN HARBINGER
- Excessive homework negatively impacts mental health, causes unnecessary stress

Caroline Lou
Opinion Editor Jula Utzschneider writes on the overwhelming chip on every student’s shoulder: homework.
Jula Utzschneider , Opinion Editor November 10, 2021
When the bell rings to end last period every day, I feel a sense of relief. However, this feeling soon wears off as I realize just how much work I have to do after the already-stressful school day ends.
While homework can be beneficial, more often than not, it is assigned excessively and unnecessarily. Teachers give a significant amount of homework, often due the next day. This causes students to spend far too much time doing such assignments and can be detrimental.
A 2013 study conducted at Stanford University found that students in top-performing school districts who spend too much time on homework experience more stress, physical health problems, a lack of balance in their lives and alienation from society. That study, published in The Journal of Experimental Education , suggested that any more than two hours of homework per night is counterproductive. However, students who participated in the study reported doing slightly more than three hours of homework every night.
And, yes, the amount of homework given to students depends on the course level they take. But, with increasingly competitive college acceptance rates (demanding more extracurriculars and college-level classes), many students feel forced to take these more challenging courses. This is a huge problem, especially as teachers give homework only thinking about their own class, not the five or six others students have.
Additionally, when it came to stress, more than 70% of students in the Stanford study said they were “often or always stressed over schoolwork,” with 56% listing homework as a primary stressor. More than 80% of students reported having at least one stress-related symptom (such as headaches, exhaustion, sleep deprivation, weight loss, stomach problems and more) in the past month, and 44% said they had experienced three or more symptoms.
Less than 1% of the students said homework was not a stressor, demonstrating that the vast majority feel overwhelmed and pressured by the amount of work they receive.
Not to mention, the time spent on these assignments could easily be spent doing something enjoyable. Many students feel forced or obligated to choose homework over practicing other talents or skills, which should never be the case. Teachers should be encouraging these extracurriculars, rather than making it impossible for students to partake in them.
In terms of what teachers can do, it’s quite simple, really. Homework is intended for students to either practice a subject further or to cover topics teachers couldn’t during the allotted class time. It should not be busywork that just wastes a student’s time.
Teachers should be giving students work that is absolutely necessary (not busy work), and eliminate it altogether where they can. It is extremely important that students not only get through high school but thrive and enjoy it too.
How much time do you spend doing homework on an average school night?
Sorry, there was an error loading this poll.
A donation of $40 or more includes a subscription to the 2023-24 print issues of The Harbinger. We will mail a copy of our fall, winter, spring and graduation issues to the recipient of your choice. Your donation supports the student journalists of Algonquin Regional High School and allows our extracurricular publication to purchase equipment and cover our annual website hosting costs.

Jula began writing for The Harbinger her freshman year after taking Journalism. While at first hesitant, she grew to love reporting and specifically opinion...

- Limitations are necessary for freedom of speech
- Mocking accents spreads unjust, offensive stereotypes
- The Great Debate: Should modern literature replace the classics in English class?
- The Director’s Eye: Heathers
- Students given too much homework, not enough time
What are you most excited for this summer?
- Going to the beach
- Pickleball 24/7
- Sleeping in
View Results
- Polls Archive

Senior Reflection: Cohorts, clubs, community

Senior Reflection: Put yourself out there

Senior Reflection: Finding my inner creator

Pop the Balloon: Inflated grades harm students

Senior Reflection: Ramblings of a senior citizen

Senior Reflection: A small high school called Algonquin

Senior Reflection: Slow down, you’re doing fine

Senior Reflection: The moments and lessons I have gathered

Senior Reflection: A little advice for freshmen; it goes way faster than you think

Senior Reflection: A love letter to Algonquin
The official student news site of Algonquin Regional High School in Northborough, MA
- Corrections
Comments (3)
Cancel reply
Your email address will not be published. Required fields are marked *
m • May 4, 2023 at 12:41 pm
m • Jan 4, 2024 at 12:10 pm
sophia • Mar 8, 2023 at 1:57 pm
very true in fact i am writing a essay right now this is resurch

- The Great Debate
- Senior Issue
- Movies & TV
- Video Games
- Pro & College
- Cafe Takeaways
- Chicken Sandwich Chasers
- Club Detectors
- Hidden Gems
- The Racer Ru-view
- Print Issues
Along with Stanford news and stories, show me:
- Student information
- Faculty/Staff information
We want to provide announcements, events, leadership messages and resources that are relevant to you. Your selection is stored in a browser cookie which you can remove at any time using “Clear all personalization” below.

Education scholar Denise Pope has found that too much homework has negative effects on student well-being and behavioral engagement. (Image credit: L.A. Cicero)
A Stanford researcher found that too much homework can negatively affect kids, especially their lives away from school, where family, friends and activities matter.
“Our findings on the effects of homework challenge the traditional assumption that homework is inherently good,” wrote Denise Pope , a senior lecturer at the Stanford Graduate School of Education and a co-author of a study published in the Journal of Experimental Education .
The researchers used survey data to examine perceptions about homework, student well-being and behavioral engagement in a sample of 4,317 students from 10 high-performing high schools in upper-middle-class California communities. Along with the survey data, Pope and her colleagues used open-ended answers to explore the students’ views on homework.
Median household income exceeded $90,000 in these communities, and 93 percent of the students went on to college, either two-year or four-year.
Students in these schools average about 3.1 hours of homework each night.
“The findings address how current homework practices in privileged, high-performing schools sustain students’ advantage in competitive climates yet hinder learning, full engagement and well-being,” Pope wrote.
Pope and her colleagues found that too much homework can diminish its effectiveness and even be counterproductive. They cite prior research indicating that homework benefits plateau at about two hours per night, and that 90 minutes to two and a half hours is optimal for high school.
Their study found that too much homework is associated with:
* Greater stress: 56 percent of the students considered homework a primary source of stress, according to the survey data. Forty-three percent viewed tests as a primary stressor, while 33 percent put the pressure to get good grades in that category. Less than 1 percent of the students said homework was not a stressor.
* Reductions in health: In their open-ended answers, many students said their homework load led to sleep deprivation and other health problems. The researchers asked students whether they experienced health issues such as headaches, exhaustion, sleep deprivation, weight loss and stomach problems.
* Less time for friends, family and extracurricular pursuits: Both the survey data and student responses indicate that spending too much time on homework meant that students were “not meeting their developmental needs or cultivating other critical life skills,” according to the researchers. Students were more likely to drop activities, not see friends or family, and not pursue hobbies they enjoy.
A balancing act
The results offer empirical evidence that many students struggle to find balance between homework, extracurricular activities and social time, the researchers said. Many students felt forced or obligated to choose homework over developing other talents or skills.
Also, there was no relationship between the time spent on homework and how much the student enjoyed it. The research quoted students as saying they often do homework they see as “pointless” or “mindless” in order to keep their grades up.
“This kind of busy work, by its very nature, discourages learning and instead promotes doing homework simply to get points,” Pope said.
She said the research calls into question the value of assigning large amounts of homework in high-performing schools. Homework should not be simply assigned as a routine practice, she said.
“Rather, any homework assigned should have a purpose and benefit, and it should be designed to cultivate learning and development,” wrote Pope.
High-performing paradox
In places where students attend high-performing schools, too much homework can reduce their time to foster skills in the area of personal responsibility, the researchers concluded. “Young people are spending more time alone,” they wrote, “which means less time for family and fewer opportunities to engage in their communities.”
Student perspectives
The researchers say that while their open-ended or “self-reporting” methodology to gauge student concerns about homework may have limitations – some might regard it as an opportunity for “typical adolescent complaining” – it was important to learn firsthand what the students believe.
The paper was co-authored by Mollie Galloway from Lewis and Clark College and Jerusha Conner from Villanova University.
Media Contacts
Denise Pope, Stanford Graduate School of Education: (650) 725-7412, [email protected] Clifton B. Parker, Stanford News Service: (650) 725-0224, [email protected]
August 22, 2022
Children’s Risk of Suicide Increases on School Days
Unlike in adults, suicide risk among children is lowest during the summer and higher during the school year. Understanding these patterns can help prevent and treat suicidality
By Tyler Black
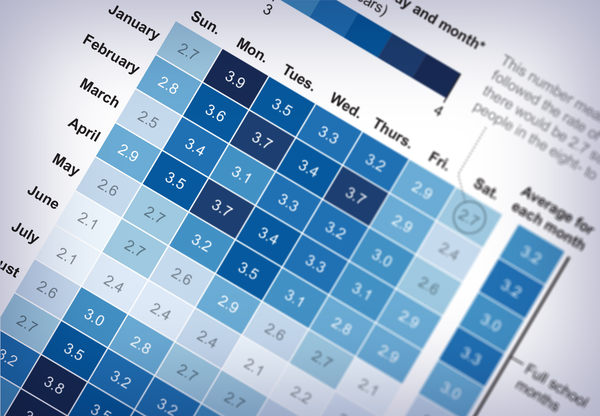
Amanda Montañez
Reading about death and suicidality can be distressing. Please read this in a moment where you feel safest and ready to do so.
Pediatricians, child psychologists and psychiatrists, social workers and pediatric emergency teams know something that many people who care for children don’t: we are much busier during the school year. I’m a full-time emergency psychiatrist who works at a major children’s hospital, and often when children come in for a mental health crisis, one of the main stressors they discuss is school.
I’m sure most people assume I commonly prescribe medications as a physician, but one of my most common “prescriptions” is advocating for reducing school burden and load. In a 2013 American Psychological Association survey, 83 percent of adolescents stated that school was a cause or significant source of stress . In a 2017 survey of school leaders in the U.K., 82 percent reported increased mental health issues among primary school children during the time of national examinations. In studies in 2013 and 2015, scientists studying homework in the U.S. found that primary school children were averaging 30 minutes of such work per night, while high-performing secondary students were averaging more than three hours per night, at the cost of their physical health and schoolwork-life balance.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Whether we are talking about referrals to mental health programs for crisis, presentations to emergency departments for mental health issues, admissions to intensive care units for urgent treatment of suicide attempts or deaths by suicide, an association with school is clear. We are able to visualize this in a number of ways.
By using the Centers for Disease Control and Prevention’s Wonder database to find information on pediatric (17 years of age or younger) deaths by suicide, I have created a “heat map” of youth suicide, and a school-day association is plain to see. On weekdays and during school months, there is a significant elevation of suicide deaths in children.
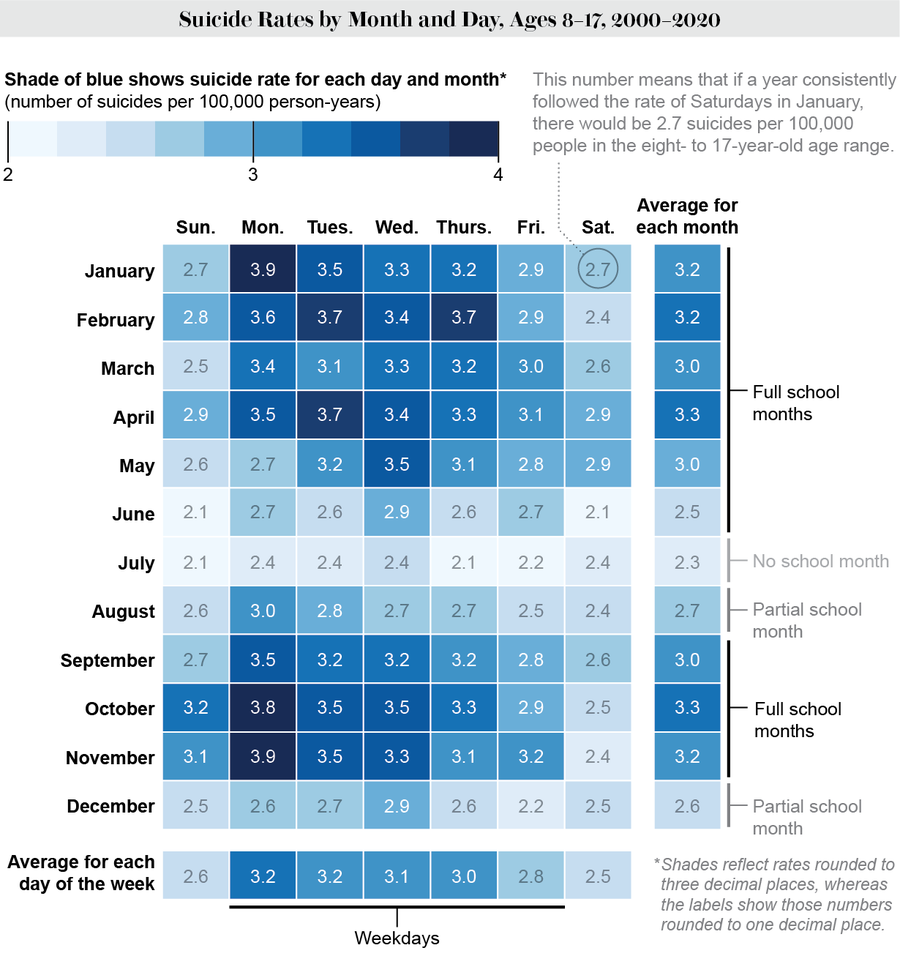
Credit: Amanda Montañez; Source: CDC Wonder , Centers for Disease Control and Prevention; Data analysis by Tyler Black
Looking at the monthly data, we can see that this elevation is not trivial: during school months, the increase in pediatric suicides ranges between 30 and 43 percent. This is in sharp contrast with adults, where we see suicide rates typically peak in summer months.
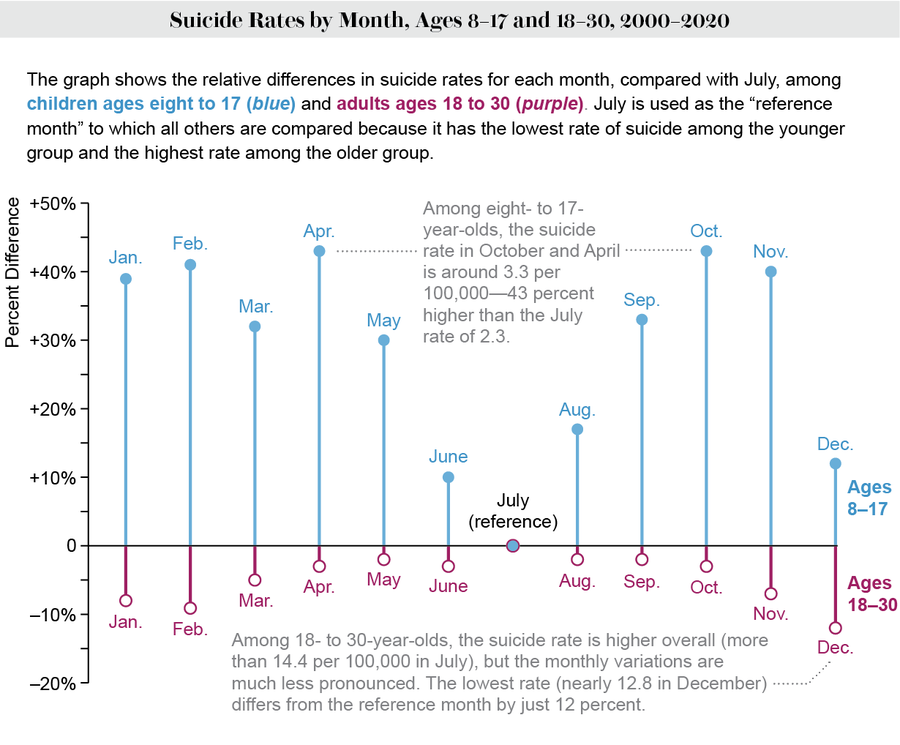
This situation has not improved over time: Compared with summer weekends, school-month weekdays from 2016 to 2019 show a pediatric suicide rate increase of 62 percent. The increase was 42% from 1999 to 2015.
If we look at far more common events, such as emergency room visits for mental health conditions, we see a strikingly similar pattern. These data come from participating hospitals in a collection done by the CDC.
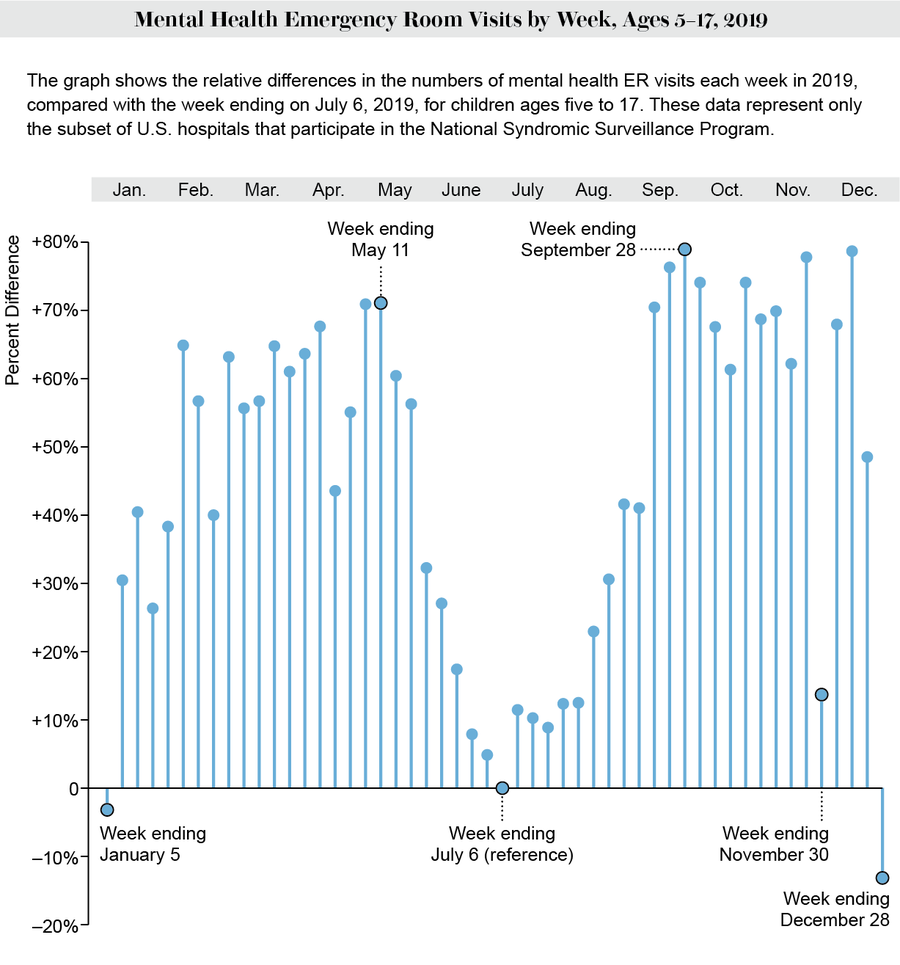
Credit: Amanda Montañez; National Syndromic Surveillance Program via Centers for Disease Control and Prevention
School comes with many things, good and bad. School can be wonderful, with learning experiences, social successes and a sense of connection to others. But it can also be incredibly stressful because of academic burden, bullying, health- and disability-related barriers, discrimination, lack of sleep and sometimes abuse. I often liken going to school to a child’s full-time job. The child has co-workers (classmates arranged by hierarchy), supervisors (teachers), bosses (administrators and principals) and overtime (homework). And they have very early work hours (most schools have hours that are very incompatible with children’s sleep patterns). Of course, work can be rewarding, but it’s also stressful.
Any time I present these data to teachers, parents, principals or school administrators, they are shocked. This should be common knowledge. Pediatric suicides and mental health crisis rates increase sharply when school is in and ease when school is out. This pattern is also found in other jurisdictions, such as Japan , Germany and Finland .
There are a number of ways to potentially mitigate this distress that I wish those responsible for our children’s education would explore. Some suggestions I’d put forth:
Reduce homework (preferably get rid of it). Some of the best educational science available shows that excessive homework is of limited benefit and in fact harms children’s health and well-being.
Add a mental health curriculum. We have developed incredible educational goals for math, reading, science and the arts. There should be a dedicated pathway for a much more universal and necessary learning: how to take care of yourself; how to look out for and help others; and how to improve both the detection and prevention of mental health crises.
Take bullying seriously and don’t just focus on the bullies. The bullied and bullies often come from similar backgrounds (histories of abuse, trauma, chaos, deprivation, parental detachment, though this certainly isn’t universally the case). But whereas the bullied tend to be internalizers, the bullies are more often externalizers. Bullies who were once victims of bullying have the highest risk of having psychiatric problems in the future.
Restore funding for playtime, music and art in school and de-emphasize academic overload. Children need relaxation, comfort, beauty, fun and play. Children who have opportunities to play and rest will learn more in their academics, and they will be able to sustain their development as they grow.
End “perfect attendance” awards and goals. While problematic truancy should be addressed, there is no less realistic notion for the rest of one’s life than the idea of “perfect attendance.” We should all, from time to time, recognize when we are at our limit and need a break. Children should be encouraged to report when they can do so and be supported.
Start school later. How many more decades of research do we need to show that children need more sleep and that adolescents do better in school when the day starts later? It’s time to make serious structural changes to the early-morning wake-up times.
Be nonjudgmental and respect children’s identity and identity formation. This is not a “woke” concept. This is a caring, compassionate concept that works for all children all the time.
Recognize and address child abuse within schools. There exist (and many readers may likely recall) teachers who are abusive, punitive and cruel. In one 2015 study, 44 percent of undergraduates recalled a time in K–12 school that they labeled as emotional abuse by a teacher . And in another study published in 2019, 3.4 percent of seventh- and eighth-grade students reported teachers bullied them .
Every year my colleagues in the emergency department brace for the coming mid-September wave, as every year our mental health crisis presentation volumes double and our days become much busier. Likely not coincidentally, in my jurisdiction, school starts in the second week of September.
In the new school year, if you are someone who works with school-age children, ask yourself what you could be doing to reduce pressure or improve quality of life for the children in your care. This would truly be suicide prevention.
IF YOU NEED HELP If you or someone you know is struggling or having thoughts of suicide, help is available. Call or text the 988 Suicide & Crisis Lifeline at 988 or use the online Lifeline Chat . LGBTQ+ Americans can reach out to the Trevor Project by texting START to 678-678 or calling 1-866-488-7386.
This is an opinion and analysis article, and the views expressed by the author or authors are not necessarily those of Scientific American .
- How It Works
- Sleep Meditation
- VA Workers and Veterans

- How It Works 01
- Sleep Meditation 02
- Mental Fitness 03
- Neurofeedback 04
- Healium for Business 05
- VA Workers and Veterans 06
- Sports Meditation 07
- VR Experiences 08
- Social Purpose 11
Does Homework Cause Stress? Exploring the Impact on Students’ Mental Health
How much homework is too much?

Homework has become a matter of concern for educators, parents, and researchers due to its potential effects on students’ stress levels. It’s no secret students often find themselves grappling with high levels of stress and anxiety throughout their academic careers, so understanding the extent to which homework affects those stress levels is important.
By delving into the latest research and understanding the underlying factors at play, we hope to curate insights for educators, parents, and students who are wondering is homework causing stress in their lives?
The Link Between Homework and Stress: What the Research Says
Over the years, numerous studies investigated the relationship between homework and stress levels in students.
One study published in the Journal of Experimental Education found that students who reported spending more than two hours per night on homework experienced higher stress levels and physical health issues . Those same students reported over three hours of homework a night on average.
This study, conducted by Stanford lecturer Denise Pope, has been heavily cited throughout the years, with WebMD eproducing the below video on the topic– part of their special report series on teens and stress :
Additional studies published by Sleep Health Journal found that long hours on homework on may be a risk factor for depression while also suggesting that reducing workload outside of class may benefit sleep and mental fitness .
Lastly, a study presented by Frontiers in Psychology highlighted significant health implications for high school students facing chronic stress, including emotional exhaustion and alcohol and drug use.
Homework’s Potential Impact on Mental Health and Well-being
Homework-induced stress on students can involve both psychological and physiological side effects.
1. Potential Psychological Effects of Homework-Induced Stress:
• Anxiety: The pressure to perform academically and meet homework expectations can lead to heightened levels of anxiety in students. Constant worry about completing assignments on time and achieving high grades can be overwhelming.
• Sleep Disturbances : Homework-related stress can disrupt students’ sleep patterns, leading to sleep anxiety or sleep deprivation, both of which can negatively impact cognitive function and emotional regulation.
• Reduced Motivation: Excessive homework demands could drain students’ motivation, causing them to feel fatigued and disengaged from their studies. Reduced motivation may lead to a lack of interest in learning, hindering overall academic performance.
2. Potential Physical Effects of Homework-Induced Stress:
• Impaired Immune Function: Prolonged stress could weaken the immune system, making students more susceptible to illnesses and infections.
• Disrupted Hormonal Balance : The body’s stress response triggers the release of hormones like cortisol, which, when chronically elevated due to stress, can disrupt the delicate hormonal balance and lead to various health issues.
• Gastrointestinal Disturbances: Stress has been known to affect the gastrointestinal system, leading to symptoms such as stomachaches, nausea, and other digestive problems.
• Cardiovascular Impact: The increased heart rate and elevated blood pressure associated with stress can strain the cardiovascular system, potentially increasing the risk of heart-related issues in the long run.
• Brain impact: Prolonged exposure to stress hormones may impact the brain’s functioning , affecting memory, concentration, and cognitive abilities.
The Benefits of Homework
It’s important to note that homework also offers many benefits that contribute to students’ academic growth and development, such as:
• Development of Time Management Skills: Completing homework within specified deadlines encourages students to manage their time efficiently. This valuable skill extends beyond academics and becomes essential in various aspects of life.
• Preparation for Future Challenges : Homework helps prepare students for future academic challenges and responsibilities. It fosters a sense of discipline and responsibility, qualities that are crucial for success in higher education and professional life.
• Enhanced Problem-Solving Abilities: Homework often presents students with challenging problems to solve. Tackling these problems independently nurtures critical thinking and problem-solving skills.
While homework can foster discipline, time management, and self-directed learning, the middle ground may be to strike a balance that promotes both academic growth and mental well-being .
How Much Homework Should Teachers Assign?
As a general guideline, educators suggest assigning a workload that allows students to grasp concepts effectively without overwhelming them . Quality over quantity is key, ensuring that homework assignments are purposeful, relevant, and targeted towards specific objectives.
Advice for Students: How to balance Homework and Well-being
Finding a balance between academic responsibilities and well-being is crucial for students. Here are some practical tips and techniques to help manage homework-related stress and foster a healthier approach to learning:
• Effective Time Management : Encourage students to create a structured study schedule that allocates sufficient time for homework, breaks, and other activities. Prioritizing tasks and setting realistic goals can prevent last-minute rushes and reduce the feeling of being overwhelmed.
• Break Tasks into Smaller Chunks : Large assignments can be daunting and may contribute to stress. Students should break such tasks into smaller, manageable parts. This approach not only makes the workload seem less intimidating but also provides a sense of accomplishment as each section is completed.
• Find a Distraction-Free Zone : Establish a designated study area that is free from distractions like smartphones, television, or social media. This setting will improve focus and productivity, reducing time needed to complete homework.
• Be Active : Regular exercise is known to reduce stress and enhance mood. Encourage students to incorporate physical activity into their daily routine, whether it’s going for a walk, playing a sport, or doing yoga.
• Practice Mindfulness and Relaxation Techniques : Encourage students to engage in mindfulness practices, such as deep breathing exercises or meditation, to alleviate stress and improve concentration. Taking short breaks to relax and clear the mind can enhance overall well-being and cognitive performance.
• Seek Support : Teachers, parents, and school counselors play an essential role in supporting students. Create an open and supportive environment where students feel comfortable expressing their concerns and seeking help when needed.
How Healium is Helping in Schools
Stress is caused by so many factors and not just the amount of work students are taking home. Our company created a virtual reality stress management solution… a mental fitness tool called “Healium” that’s teaching students how to learn to self-regulate their stress and downshift in a drugless way. Schools implementing Healium have seen improvements from supporting dysregulated students and ADHD challenges to empowering students with body awareness and learning to self-regulate stress . Here’s one of their stories.
By providing students with the tools they need to self-manage stress and anxiety, we represent a forward-looking approach to education that prioritizes the holistic development of every student.
To learn more about how Healium works, watch the video below.
About the Author

Sarah Hill , a former interactive TV news journalist at NBC, ABC, and CBS affiliates in Missouri, gained recognition for pioneering interactive news broadcasting using Google Hangouts. She is now the CEO of Healium, the world’s first biometrically powered immersive media channel, helping those with stress, anxiety, insomnia, and other struggles through biofeedback storytelling. With patents, clinical validation, and over seven million views, she has reshaped the landscape of immersive media.
- Close Menu Search
- Award Winners

Is Excessive Homework the Cause of Many Teen Issues?
Sydney Trebus , Business Manager | September 15, 2019
Does excessive homework really make a student perform worse? Is homework a big influencer on the emotional and physical health of students? Can we change the bad reputation homework has obtained over the years or is it too late?
Today, schooling is ever-changing, currently focusing on a “necessary” end goal of attending college. Standards are rising, teachers are better trained, and students are left with rigorous courses riddled with hours and hours of homework. People are now wondering how important homework really is. Is that just the overload talking or does homework actually have a negative impact on students?
Popular opinion would suggest yes, claiming that homework is a useless and stress-inducing part of school at any age. Many Boulder High students communicate a similar complaint.
Seniors Carson Williams and Carson Bennett voiced their opinions. Bennet says that “Homework results in later bedtimes which means we get less sleep and therefore, have less energy the next day.” Williams agreed and added,“Homework is good if you need it to study, but if it is just busywork then it is useless.”
Another student, Bishal Ellison, commented that in some classes “homework doesn’t impact [his] success, there is no point … In one of [his] classes, homework is just for extra credit.”
While student opinions are extremely significant, teachers are the ones in control of this so-called “stress inducing and useless activity.”
Mr. Weatherly, an AP World Geography teacher here at Boulder High, commented that homework has an enormous impact on the success of students within the class; he claimed that there is simply not enough time in class to review everything. He does, however, agree with popular opinion, saying, “Teachers give homework thinking about their own class, not the five or six others students have.”
So which is it? How important is homework? Homework has been seen both beneficial and detrimental in association with time. Homework over a certain time limit can cause stress, depression, anxiety, lack of sleep, and more.
Homework distracts from extracurriculars and sports as well, something colleges often look for. Homework is ultimately leading students to resent school as a whole.
According to a study done by Stanford University, 56 percent of students considered homework a primary source of stress, 43 percent viewed tests as a primary stressor, while less than one percent of the students said homework was not a stressor. They were able to conclude that too much homework can result in a lack of sleep, headaches, exhaustion, and weight loss.
Experts denote that the homework assigned to students today promotes less active learning and instead leads to boredom and a lack of problem-solving skills. Active learning, done through students learning from each other through discussion and collaboration, enhances a student’s ability to analyze and apply content to aid them in a real-world setting.
This negative attitude towards homework can, unfortunately, arise at a young age, especially in today’s schooling systems.
Students in all grades are required to extend the hard rigor of school past the average eight hours they need to spend inside the building. According to an Education Week article by Marva Hinton, kindergarteners are often required to do a minimum of 30 minutes of homework a night; these young students are expected to read for 15 minutes as well as work on a packet for another 15-30 minutes.
Kindergarten is forcing children to learn concepts they may not be ready for, discouraging them at a young age. As a principle rule, the National PTA recommends 10 to 20 minutes of homework per night for children in first grade and an additional 10 minutes for every grade after that.
After this time marker, homework begins to be detrimental to the success of a student. Additionally, according to the Journal of Educational Psychology , students who did more than 90 to 100 minutes of homework per night actually did worse on tests than those with less than 90 minutes of homework.
The hours of homework students receive takes time that could be spent on extracurriculars, with family and friends, or on sports or activities. Children and young adults focus a large part of their time and energy on school, removing time to replenish and work on other skills in life, including socializing.
Physical activity can actually be very beneficial to the success rates of students, improving self-esteem, well-being, motivation, memory, focus, and higher thinking.
According to the US Center for Disease Control and Prevention (CDC ) , exercise has an impact on cognitive skills such as concentration and attention, and it enhances classroom attitudes and behaviors.
The more time taken away from the emotional and physical health of a student, the more resentful they will be towards school. In kindergarten, over 85 percent of students are enthusiastic about learning and attending school, whereas 40 percent of high school students are chronically disengaged from school and any learning that takes place.
What’s even more baffling is that as students enter high school, they are expected to be enthusiastic about school, obtain perfect grades and test scores, and do extracurricular activities and sports in order to get into a good college.
Logan Powell, the Dean of Admissions at Brown University asks when accepting students, “Have they learned time management skills, leadership, teamwork, discipline? How have they grown as a person and what qualities will they bring to our campus?”
These are unrealistic standards for students who most likely already have negative attitudes towards school and homework and aren’t given the opportunity to work on the skills colleges look for by exploring their community through clubs, volunteering, and working.
Experts see how detrimental homework can really be for a plethora of reasons; Donaldson Pressman reported that homework is not only not beneficial to a students grades or GPA, but it is also detrimental to their attitude towards school, their grades, their self-confidence, their social skills, and their quality of life.”
Homework, however, helps student achievement, reinforces good habits, involves parents in their students’ learning, and helps students remember material learned in class.
This is all based on the circumstances however, if schools keep making homework more prominent in the learning system, students will lose their passion for learning. Unfortunately, many of us already have. So when teachers consider giving homework to their students, they should ask themselves how they believe it will improve their students’ learning and abilities.

Clash of Clans: In Defense of “Rushing”

Casa Bonita Makes a Splash
Instagram Turns Us Into Awful People

Pecking Away at Food Waste

The Glamorization of Mental Illness
Comments (4)
Cancel reply
Your email address will not be published. Required fields are marked *
Chris • Feb 23, 2023 at 6:24 am
I hate homework in 5th grade
Jason • May 24, 2023 at 8:25 am
good luck in 7th grade then cuz that’s where finals start to get difficult. I’m barely keeping up right now and have to stay up late to keep up with the work.
John • May 9, 2022 at 11:40 am
Good article very informative.
Garrett • May 19, 2022 at 7:01 pm
yes i agree
4 Ways Parents Can Deal With Summer Homework, According to Experts Say
Most schools assign summer homework with good intentions, but they don't always know how to make school-break assignments meaningful.

School’s out for summer! Around the country, students have chucked their backpacks and planners aside and rejoiced. That is, if they don’t have summer homework.
A hotly debated topic in education, summer assignments can involve reading, online work, packets, and/or real-life enrichment opportunities in communities that students are responsible for completing by the time school resumes. It’s become a burden for some families whose parents work in the summer, or who lack teacher support or internet access. On the other hand, some parents want their children doing summer work to keep them busy and engaged in academics, and to prevent the “summer slide” — a regression in learning some educators believe occurs between school years.
Licensed Psychologist Connie McReynolds , Ph.D., says summer work can sometimes cause children to feel like they’re still at school. “It can lead to burnout before the next school year begins,” she says. For others, she says, the structure and routine are beneficial.
So summer homework can be advantageous — if it’s done right. The bad news is that, in a lot of cases, it isn’t. Here’s what the experts had to say about if, when and how summer work should be assigned — and how parents can cope if their school is missing the mark.
When Summer Homework Is Done Right
It should be intentional and (actually) educational..
“Summer work for the sake of raising and/or setting expectations for rigor is baseless,” Davis says. “Students often put off the work until the last minute and complete the work for compliance, not true learning. And that’s only exacerbated when the teachers don’t create a meaningful classroom connection to the summer work.” This points to a problem with practices around all homework — are they meaningful practice, or just a check-the-box completion grade?
Teachers might feel they can’t teach all the material during the school year. But a 2023 study found that summer learning had a small impact on math test scores for students but not reading. Additional recent data has shown that the impact of the “summer slide” depends on a variety of factors, including grade and poverty levels.
What parents can do : “The teacher should provide a clear connection to how the summer work is going to enhance the learning and/or enrich the learning that will occur at the start of the year,” Davis says. “If there isn’t a clear explanation of the purpose of the summer work, parents should reach out to the teacher directly for clarity regarding the purpose of the work and if it is required." Don’t worry about being a nudge. “Parents should keep in mind they are advocates for their children and asking questions for clarity creates a two way dialogue with the teacher,” she adds.
It should come with tech and academic support.
A key pillar of homework is homework help — that is, if the purpose is real learning.
Many parents can probably relate to a scenario like this: “Hey mom, I’m supposed to work on a school app called blah blah blah.”
“Oh, okay, what’s the password?”
“I don’t know.”
And even if they can log in, what happens if kids don’t understand the assignments? Many parents can relate to not knowing the answer to a homework question a kid is asking, and not knowing which resources to use to find it. Adding in homework help around work hours can add stress to a family.
Not a whole lot of learning is happening in these situations, which all lead back to one missing aspect to effective homework practices — teacher support. Teachers are off in the summer, but if students aren’t, there’s an issue with technical troubleshooting and guided instruction.
“Homework should reinforce skills learned in the classroom,” Davis says. “Unfortunately all too often students are left to complete homework without the foundational knowledge to complete it to enhance their learning. During the summer months teachers are typically not available leaving the students to complete the homework with little to no direction which could result in them replicating bad habits without any checkpoints or feedback.”
What parents can do : It’s absolutely reasonable to expect summer support to have necessary technology and instructional guidance, even in the summer. “Students should be able to access the teacher to provide clarity, answer questions and/or to provide feedback,” Davis says. She again recommends communicating with the school as early as possible about how students are supposed to get tech or instructional support.
It should be inclusive and low-stress.
A student with an Individualized Education Plan, or a 504 plan, who typically has extra homework time looks at a large packet at the start of summer. Do they still have double time? What resources are available to them? These are concerns that all families, but especially those with additional academic and learning needs, have to navigate.
“Parents of children with ADHD are naturally concerned about whether being away from academic studies over the summer will lead to the ‘summer slide,’” McReynolds says. “This concern leads parents to struggle with whether to push on through the summer or give children a break from the pressure.”
Students who don’t have access to support can see an increase in academic-related stress too. According to a 2021 study by Challenge Success, a non-profit organization affiliated with the Stanford University Graduate School of Education, 56% of students reported an increase in stress from school . The same report found that during the school year, students spent an average of three hours on homework each weeknight, with 51% reporting they spent more time on homework than they did in the past. But 42% reported they had a decreased level of engagement for school and learning. So, experts are torn on whether homework actually increases engagement, and even learning.
“All too often the completion or lack thereof is utilized to gatekeep students out of higher level courses,’ Davis says. “In the event a student faces this, parents need to actively advocate for inclusion in the class regardless of completion of the summer work.”
What parents can do: “Individual accommodations and modifications included in a student’s IEP/504 must be taken into account,” Davis says. “Another approach to summer work would be for the parent and student to create a scaffolded schedule to complete the work as opposed to waiting until the final weeks of summer to complete it all at once. Ultimately, the mental health of the student is most important and parents and/or the student should actively communicate with the teacher directly to discuss concerns throughout the summer.”
High schoolers who are taking Advanced Placement (AP) classes, which sometimes require summer work, can consider opting for a College Credit Plus (CPP) class, when appropriate for them. CPP classes often carry the same weight without the summer work, but it varies state to state, and parents and students should ensure the desired university they would like to attend accepts CPP classes as credit as they do with AP. Pro tip from Davis: Ask around or ask the teacher before April or May to determine summer homework plans for an AP class, because you might miss the deadline to do CPP if you wait until summer.
It should even be…fun!
There just might be room in summer homework for a bit of enjoyment, with the right set up.
“I believe summer homework is detrimental for several reasons,” Davis says. “It perpetuates burnout … preventing students from fully relaxing and recharging during their break. This can negatively impact their mental health and overall well-being.” So, the only summer homework our experts are interested in are fun activities that enrich family or community life, or personal development.
Emily Pendergrass , associate professor of the Practice of Literacy and Reading Education at Vanderbilt University says summer homework should be meaningful for families, teachers and learning. “It should be interactive,” she says. “It shouldn’t be one size fits all…we should be moving towards learning and curiosity.”
Summer homework should move into meaningful activities, Pendergrass says. For example, instead of keeping a reading log that just lists the titles of books and how many minutes were read, students can be tasked with drawing a picture of what they read, writing an alternate ending, or making a short video about the reading to share with classmates when they’re back to school.
What parents can do: In the end, there’s no faster way to get students to hate school than assigning a classic piece of literature, and telling them good luck, see you in the fall. Pushback from parents, community and students themselves can ensure summer work, if necessary, is equitable and purposeful, well-supported and inclusive. Or, we can just cut it all together and go read something fun by the pool…
When to Call It Off
If your child is too stressed about summer homework, you and your child, and their educators, can discuss together if the right move is to simply not do it . What are the consequences? The ramifications of this depend on the school, and the program. In some places, summer work might not account for a large portion of their final grade and a student might be confident they can make it up during the school year. In others, they might be able to choose a less rigorous course without a summer homework requirement. Then again, skipping summer homework might result in failing a class if the summer assignments are weighted heavily in the final grade. You can also consider asking for an alternative or makeup assignment, which often would be considered on a case-by-case basis. “If summer work is being graded on completion, and not truly being utilized at the start of the year to extend instruction, the student, parent and teacher need to actively discuss the true purpose of the work,” Davis says.
Alexandra Frost is a Cincinnati-based freelance journalist and content marketing writer, focusing on health and wellness, parenting, education, and lifestyle. She has been published in the Atlantic , Glamour , Today’s Parent , Reader’s Digest , Consumer Reports , Women’s Health , and National Geographic . She spends her “free” time with her five kids under age 8, and testing lots of products. To connect or read more of her work please visit alexandra-frost.com or follow her on social media: Twitter Instagram Linked In .

@media(max-width: 64rem){.css-o9j0dn:before{margin-bottom:0.5rem;margin-right:0.625rem;color:#ffffff;width:1.25rem;bottom:-0.2rem;height:1.25rem;content:'_';display:inline-block;position:relative;line-height:1;background-repeat:no-repeat;}.loaded .css-o9j0dn:before{background-image:url(/_assets/design-tokens/goodhousekeeping/static/images/Clover.5c7a1a0.svg);}}@media(min-width: 48rem){.loaded .css-o9j0dn:before{background-image:url(/_assets/design-tokens/goodhousekeeping/static/images/Clover.5c7a1a0.svg);}} Parenting Tips & Advice

150 Hispanic Girl Names for 2024

200 Unique Middle Names for Girls

The Ultimate Back-to-School Supplies List

Where Can Teens Go?

115 Cute Hispanic Baby Boy Names

Fresh Dad Jokes for Corny Parents

Can You Solve These Tricky Riddles for Kids?

Unique Ways to Announce Your Pregnancy
Empty Nesters Need Support, Too
How to Stay Connected to Your Screen-Addicted Teen

50 Simple Fall Crafts for Kids
Mass IT outage hits airports, businesses and broadcasters around the world
Major airlines, medical facilities, businesses and police forces around the world were hit by a massive information technology glitch Friday, with Microsoft computers around the world showing " Blue Screens of Death ."
The cybersecurity company CrowdStrike said Friday that the outages were the result of a routine software update gone wrong, "not a security incident or cyberattack."
CrowdStrike, which provides cybersecurity services and software for many large corporations that use Microsoft systems, later issued a new software update that automatically fixed some computers. But others must be manually restarted and patched, causing huge delays.
Microsoft announced late Friday morning that its 365 apps and services had recovered, and CrowdStrike said a fix had been deployed early in the morning. But some frozen computers couldn’t receive CrowdStrike’s automatic update, leading to some of the problems lingering into the weekend.
Many flights were delayed as of Friday afternoon . Starbucks locations in New York had resumed normal wait times , despite its mobile order-ahead feature still not working.
The glitch brought chaos to a number of key institutions and businesses around the world that may take some time to clear up.
Many flights were grounded across the globe, and stores and broadcasters in several countries went offline. According to the aviation technology company FlightAware, the tech glitch was responsible for more than half of the U.S.'s 1,352 flight delays and cancellations before 8 a.m. ET on Friday.
Major carriers, including American Airlines , Delta Air Lines and United Airlines , all issued ground stops Friday morning citing communications issues. Passengers traveling to the United States from as far away as Japan had their flights canceled. Delta ordered a “global ground stop,” said Rep. Eric Swalwell, D-Calif., a member of the House subcommittee on cybersecurity.
Follow live updates on the global tech outage
George Kurtz, president and CEO of CrowdStrike, said the problems could persist.
“It could be some time for some systems that just automatically won’t recover,” he told NBC’s “TODAY” show on Friday.
Kurtz said the company was “deeply sorry for the impact that we’ve caused to customers, to travelers, to anyone affected by this,” adding the issue had been fixed on its end.
“Many of the customers are rebooting the system, and it’s coming up, and it’ll be operational because we fixed it on our end,” he said. “We’re just trying to sort out where the negative interaction was,” he said of the faulty update that affected Windows PCs.
Earlier, in a post on X , Kurtz said that the outages were due to a “defect found in a single content update for Windows hosts.”
In Europe, Berlin’s Brandenburg Airport said there would be delays to passenger check-ins “because of a technical fault,” and Aena, which manages 46 airports in Spain, said “an incident in the computer system” could cause delays. Several budget airlines in South Korea reported technical problems and delays, The Associated Press said. Sydney Airport, one of Australia’s largest, said there would be delays.
Paris' airport authority said in a statement that while its systems were not affected ahead of next week's Olympic Games opening ceremony, "this situation has an impact on the operations of airlines at Paris-Charles de Gaulle and Paris-Orly airports: delays in check-in, delays and temporary suspension of some flights," according to the AP.

The Paris Olympics organizing committee told Reuters that while the outage was slowing its operations, its ticket sales remained unaffected. Elsewhere in the world of sports, British soccer team Manchester United delayed a ticket release scheduled for Friday, blaming problems with Microsoft.
That was just one of many businesses affected across the United Kingdom, with train operators blaming IT outages for cancellations Friday morning and the London Stock Exchange saying that a “3rd party global technical issue” had prevented its regulatory news service from posting any new items.
The country’s National Health Service posted on X that “the NHS is aware of a global IT outage and an issue with a [general practitioner] appointment and patient record system.” The NHS that the system’s emergency phone service was still operating.
In Israel, at least 15 major hospitals were affected, according to media there. However, most medical centers had either returned to normal operations or reverted to manual operations. The emergency line of the country’s ambulance service was also affected.
At 2:20 a.m. ET, Alaska State Troopers said that 911 and nonemergency phone numbers across the state were not working “due to a nationwide technology-related outage."
The glitch affected news outlets, too, including NBC News. Sky News, NBC News' British partner broadcaster, was temporarily unable to air live news. Broadcasters in Australia also reported problems.
In a blog posted late Friday afternoon , Kurtz wrote that CrowdStrike staffers would be on call over the weekend to help affected customers, and warned of hackers and scammers exploiting the situation.
“We know that adversaries and bad actors will try to exploit events like this," he said. "I encourage everyone to remain vigilant and ensure that you’re engaging with official CrowdStrike representatives."
I cover early morning U.S. breaking news, everything from severe weather to crime. I'm based in London and have worked for American news outlets since 2013.
Kevin Collier is a reporter covering cybersecurity, privacy and technology policy for NBC News.
After massive IT outage for airlines and health care, officials see signs of recovery
Federal officials expect transportation systems will largely return to normal operations by Saturday, following a massive IT outage for Windows users that knocked out systems for transportation, delivery and health care.
- Buttigieg says transportation systems returning to normal but issues remain
- What caused the Windows outages affecting flights, companies around the world
- Health services and hospitals impacted by outage
Here's what to know:
Here's what to know, live coverage contributors 30.

9:27 p.m. EDT 9:27 p.m. EDT
8:12 p.m. EDT 8:12 p.m. EDT
7:04 p.m. EDT 7:04 p.m. EDT
5:15 p.m. EDT 5:15 p.m. EDT
4:30 p.m. EDT 4:30 p.m. EDT
4:20 p.m. EDT 4:20 p.m. EDT
3:58 p.m. EDT 3:58 p.m. EDT
3:26 p.m. EDT 3:26 p.m. EDT
2:46 p.m. EDT 2:46 p.m. EDT
2:24 p.m. EDT 2:24 p.m. EDT
2:09 p.m. EDT 2:09 p.m. EDT
2:08 p.m. EDT 2:08 p.m. EDT
1:57 p.m. EDT 1:57 p.m. EDT
1:32 p.m. EDT 1:32 p.m. EDT
1:25 p.m. EDT 1:25 p.m. EDT
1:00 p.m. EDT Key update 1:00 p.m. EDT
12:59 p.m. EDT 12:59 p.m. EDT
12:38 p.m. EDT 12:38 p.m. EDT
12:20 p.m. EDT 12:20 p.m. EDT
12:11 p.m. EDT 12:11 p.m. EDT
12:00 p.m. EDT 12:00 p.m. EDT
11:32 a.m. EDT 11:32 a.m. EDT
11:29 a.m. EDT Key update 11:29 a.m. EDT
11:20 a.m. EDT 11:20 a.m. EDT
10:58 a.m. EDT 10:58 a.m. EDT
10:57 a.m. EDT 10:57 a.m. EDT
10:05 a.m. EDT 10:05 a.m. EDT
9:58 a.m. EDT 9:58 a.m. EDT
9:48 a.m. EDT 9:48 a.m. EDT
9:46 a.m. EDT 9:46 a.m. EDT
9:06 a.m. EDT 9:06 a.m. EDT
9:00 a.m. EDT 9:00 a.m. EDT
8:58 a.m. EDT 8:58 a.m. EDT
8:29 a.m. EDT Key update 8:29 a.m. EDT
8:14 a.m. EDT 8:14 a.m. EDT
8:00 a.m. EDT 8:00 a.m. EDT
7:46 a.m. EDT 7:46 a.m. EDT
7:35 a.m. EDT 7:35 a.m. EDT
7:27 a.m. EDT 7:27 a.m. EDT
7:17 a.m. EDT 7:17 a.m. EDT
7:15 a.m. EDT Key update 7:15 a.m. EDT
7:10 a.m. EDT Key update 7:10 a.m. EDT
7:00 a.m. EDT 7:00 a.m. EDT
6:53 a.m. EDT 6:53 a.m. EDT
6:41 a.m. EDT 6:41 a.m. EDT
6:32 a.m. EDT Key update 6:32 a.m. EDT
6:19 a.m. EDT 6:19 a.m. EDT
6:01 a.m. EDT Key update 6:01 a.m. EDT
5:49 a.m. EDT 5:49 a.m. EDT
5:43 a.m. EDT 5:43 a.m. EDT
5:42 a.m. EDT Reporting from London 5:42 a.m. EDT
5:41 a.m. EDT 5:41 a.m. EDT
5:40 a.m. EDT 5:40 a.m. EDT
5:31 a.m. EDT Key update 5:31 a.m. EDT
5:25 a.m. EDT 5:25 a.m. EDT
5:15 a.m. EDT 5:15 a.m. EDT
5:07 a.m. EDT 5:07 a.m. EDT
4:45 a.m. EDT Reporting from Charles de Gaulle airport 4:45 a.m. EDT
4:35 a.m. EDT 4:35 a.m. EDT
4:34 a.m. EDT 4:34 a.m. EDT
4:31 a.m. EDT 4:31 a.m. EDT
4:18 a.m. EDT 4:18 a.m. EDT

Major US carriers restore some flight operations amid global cyber outage
- Medium Text

Sign up here.
Reporting by Shivansh Tiwary, Maria Ponnezhath and Shivani Tanna ; Additional reporting by Chandni Shah, Nathan Gomes, Surbhi Mishra, Angela Christy and David Shepardson; writing by Abinaya Vijayaraghavan and Shubham Kalia; Editing by Nivedita Bhattacharjee and Sriraj Kalluvila
Our Standards: The Thomson Reuters Trust Principles. , opens new tab

CrowdStrike says significant number of outage-affected devices back up
Cybersecurity firm CrowdStrike said on Sunday that a significant number of the 8.5 million Microsoft devices that were impacted as a result of a global tech outage related to a software update were back online and operational.

Advertisement
Biden Says He’d Consider Dropping Out if a ‘Medical Condition’ Emerged
President Biden has said none of his doctors have told him he has a serious medical condition. But his debate performance last month raised questions about his health and acuity.
- Share full article

By Michael D. Shear
Reporting from Washington
- Published July 17, 2024 Updated July 21, 2024, 2:44 p.m. ET
President Biden said he would no longer seek re-election and endorsed Vice President Kamala Harris. Follow live updates.
President Biden said in an interview released on Wednesday that he would re-evaluate whether to stay in the presidential race if a doctor told him directly that he had a medical condition that made that necessary.
Mr. Biden has said repeatedly that none of his doctors have told him he has a serious medical condition. Dr. Kevin O’Connor, the White House physician, wrote after the president’s physical in February that Mr. Biden is “a healthy, active, robust, 81-year-old male who remains fit to successfully execute the duties of the presidency.”
But his disastrous performance at a debate with former President Donald J. Trump last month, which prompted a wave of calls by Democratic allies to step aside, raised questions about his health and acuity.
In an interview with Ed Gordon of BET News, Mr. Biden was asked if there was anything that would make him re-evaluate staying in the race.
“If I had some medical condition that emerged, if somebody, if doctors came to me and said, you got this problem and that problem,” Mr. Biden said.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .


IMAGES
VIDEO
COMMENTS
Health Hazards of Homework. Pediatrics. A new study by the Stanford Graduate School of Education and colleagues found that students in high-performing schools who did excessive hours of homework "experienced greater behavioral engagement in school but also more academic stress, physical health problems, and lack of balance in their lives.".
• Reductions in health: In their open-ended answers, many students said their homework load led to sleep deprivation and other health problems. The researchers asked students whether they experienced health issues such as headaches, exhaustion, sleep deprivation, weight loss and stomach problems.
Emmy Kang, mental health counselor at Humantold, says studies have shown heavy workloads can be "detrimental" for students and cause a "big impact on their mental, physical and emotional health ...
Key points. Mental health challenges and neurodevelopmental differences directly affect children's ability to do homework. Understanding what difficulties are getting in the way—beyond the usual ...
But they were not more invested in the homework itself. They also reported greater academic stress and less time to balance family, friends and extracurricular activities. They experienced more physical health problems as well, such as headaches, stomach troubles and sleep deprivation. "Three hours per night is too much," Galloway says.
In most cases, that stress is from academics, not social issues or bullying, the poll found. (See the full results here .) Homework was a leading cause of stress, with 24 percent of parents saying ...
problems in the long run, like anxiety and depression. And for all the distress homework causes, it's not as useful as many may think, says Dr. Nicholas Kardaras, a psychologist and CEO of Omega
Research shows that excessive homework leads to increased stress, physical health problems and a lack of balance in students' lives. And studies have shown that more than two hours of daily homework can be counterproductive, yet many teachers assign more.. Homework proponents argue that homework improves academic performance. Indeed, a meta-analysis of research on this issue found a ...
Elementary school kids are dealing with large amounts of homework. Howard County Library System, CC BY-NC-ND. One in 10 children report spending multiple hours on homework. There are no benefits ...
In addition to homework reducing opportunities for play, it also leads to increased conflicts and stress for families. For example, research finds that children with more hours of homework experience more academic stress, physical health problems, and lack of balance in their lives (18).
Their findings were troubling: Research showed that excessive homework is associated with high stress levels, physical health problems and lack of balance in children's lives; 56% of the ...
Lack of sleep. One of the most prevalent adverse effects of schoolwork is lack of sleep. The average student only gets about 5 hours of sleep per night since they stay up late to complete their homework, even though the body needs at least 7 hours of sleep every day. Lack of sleep has an impact on both mental and physical health.
Negative Effects of Homework for Students. While some amount of homework may help students connect to their learning and enhance their in-class performance, too much homework can have damaging effects. Students with too much homework have elevated stress levels. Students regularly report that homework is their primary source of stress.
Chinese schoolgirl uses robot to do her homework. Emmy Kang, mental health counselor at Humantold, says studies have shown heavy workloads can be "detrimental" for students and cause a "big ...
The short answer is that depression can absolutely lead to depression for a variety of reasons. Identifying the cause of your homework-related depression below is the first step toward bettering your mental health. Excessive homework, lack of social support, and perfectionism are just a few of the reasons homework can lead to depression.
This causes students to spend far too much time doing such assignments and can be detrimental. A 2013 study conducted at Stanford University found that students in top-performing school districts who spend too much time on homework experience more stress, physical health problems, a lack of balance in their lives and alienation from society.
* Reductions in health: In their open-ended answers, many students said their homework load led to sleep deprivation and other health problems. The researchers asked students whether they ...
This situation has not improved over time: Compared with summer weekends, school-month weekdays from 2016 to 2019 show a pediatric suicide rate increase of 62 percent. The increase was 42% from ...
In a poll, 56 percent of students attributed any stress in their lives to too much homework. Less than 1 percent of students said that homework was not a stressor. When asked how homework affects them, students reported lack of sleep, headaches, exhaustion and stomach problems. Students reported that due to the necessity of keeping their grades ...
Over the years, numerous studies investigated the relationship between homework and stress levels in students. One study published in the Journal of Experimental Education found that students who reported spending more than two hours per night on homework experienced higher stress levels and physical health issues.Those same students reported over three hours of homework a night on average.
Homework over a certain time limit can cause stress, depression, anxiety, lack of sleep, and more. Homework distracts from extracurriculars and sports as well, something colleges often look for. Homework is ultimately leading students to resent school as a whole. According to a study done by Stanford University, 56 percent of students ...
What parents can do: "The teacher should provide a clear connection to how the summer work is going to enhance the learning and/or enrich the learning that will occur at the start of the year ...
The country's National Health Service posted on X that "the NHS is aware of a global IT outage and an issue with a [general practitioner] appointment and patient record system." The NHS that ...
Microsoft said it was aware of an issue impacting Windows running CrowdStrike technology. American Airlines, United and Delta grounded flights.
Health care systems were crippled, forcing hospitals to cancel noncritical surgeries. In the United States, 911 lines went down in multiple states, though many of those problems were being ...
Top U.S. carriers including Delta Air and United Airlines are restoring some operations on Friday after a technical issue related to an IT vendor forced multiple carriers to ground flights.
President Biden has said none of his doctors have told him he has a serious medical condition. But his debate performance last month raised questions about his health and acuity.
The company said it determined the cause and is working to fix it. A company spokesman did not immediately respond to a request for comment. Other airlines were unaffected by the outage.
Forty-three percent viewed tests as a primary stressor, while 33 percent put the pressure to get good grades in that category. Less than 1 percent of the students said homework was not a stressor. • Reductions in health: In their open-ended answers, many students said their homework load led to sleep deprivation and other health problems. The ...
Dr. Ackerman led a study looking into the effects of energy drinks on the heart. He warns that while energy drinks may not directly cause cardiac arrest, consuming them, especially in large quantities, can be a "perfect storm" for people with genetic heart conditions. "Maybe you've been sleep deprived, and now you're taking the energy drink.