

How to Write a Nursing Reflective Essay (Guide for Nurse Students)

If you are a nursing or medicine student, you are aware that you will come across or have already come across assignments requiring you to write a nursing reflection essay. At first, such a task always appears challenging, but given the understanding of the steps, things flat out, and you can write reflective essays and get better grades.
Reflective practice is highly encouraged in nursing. Reflection entails making sense of situations, events, actions, and phenomena in the workplace.
As a nursing student, you will be asked to write a reflective essay on your clinical placement, practicum, shadowing experience, shadow health DCE activities, personal nursing philosophy, why you want to become a nurse, nursing program, ethical dilemma, knowledge, skills, and abilities, systems, and processes.
The easiest way to complete the reflective essay assignment is by first determining what reflective writing entails, its significance, its steps, and some of the best tips that form the core of this ultimate guide.
Basics of Reflective Writing in Nursing
Reflective writing is an analytical writing practice where the writer describes a real or imaginary event, scene, phenomenon, occurrence, or memory, including their takeaway. It entails the critical analysis of an experience, including recording how it has impacted you and what you intend to do with the new knowledge or how to act when such an occurrence recurs.
As you document the encounter, you can use first-person pronouns and write subjectively and objectively. This means that you can decide to either use personal experiences alone or support these experiences using citations from scholarly sources.
When writing a reflective essay in nursing, you must recount the events and give critical detail of how the events shaped your knowledge acquisition. Reflection helps nursing students develop skills in self-directed learning, which is directly associated with high motivation and improved quality of care .
In most cases, reflection occurs on what went well and what went wrong. It could be a successful operation, a thank you note from a patient, a patient who regained their health faster, or a new nursing care plan that worked. However, it can also be about adverse events such as death, postoperative complications, death of an infant at birth, dissatisfied patient, medical error, or a failed procedure.
As a nursing student, when you learn to reflect on situations, you grow to become a professional nurse who diligently does their noble duty.
When writing a reflective essay, you begin by setting the scene (explaining what, where, how, and who-the situation), detailing how you felt (emotional state), why it happened (making sense of the situation), critical review and development of insights, a note on what was learned, and strategies to address future recurrence.
Your professor may ask you to write a nursing reflective paper about various topics in your course or your experience working in a group, how you solved a problem, a healthcare issue, or clinical practice. Consider the following example of a reflective statement in nursing; in my clinical practices, I realized I focused more on the technical aspects but failed to explain what it was doing to improve their health. I would like to understand more about listening to patience and their concerns to better care for them.
As you will notice later, these reflective stages are structured into different reflective models and frameworks that we will explore in-depth. So, with the understanding of what comprises reflective writing and its importance in nursing, let's now get solid on the structure.
Related Reading:
- Philosophy of Nursing Example.
- Ideas and topics for nursing capstone or project papers
- How to write a SOAP note paper
- Top nursing debate topics
- Nursing Theories and Theorists.
Structure of a Reflective Essay in Nursing
A reflective essay is an analytical writing piece describing and evaluating encounters or experiences. When asked to write one, you should know that an excellent reflective essay consists of different parts, just like a typical academic essay. It comprises the cover or title page, introduction, body paragraphs, conclusions, and a references page.
The title page contains information about the assignment. If you are writing the reflective essay in APA, include these on the title page:
- Title of the reflective essay
- Course code and name
- Instructors name
- Name of your institution
- Date of submission
When writing in Harvard format, the title or the cover page will consist of the following:
- Title of the essay in title case and the page number (upper right margin),
- Title of the essay in CAPS,
- Name of class or course,
- Name of the instructor,
- Name of your school,
- City and state where your school is located and,
- The date of submission.
Introduction
The introduction begins with an attention grabber or a hook sentence to attract readers' attention. It should then explain the essay's purpose and signpost the ideas that will come later in the essay. The introduction also has a thesis statement at the end of the paragraph- the last sentence. The thesis is concise, clear, and relatable and should reflect your position.
Body Paragraphs
The body paragraphs of a reflective essay can be three or more, depending on the length of the essay. Essentially, the body comprises 80% of the total word count.
The first paragraph is where you describe the situation, including the events, why they occurred, how they occurred, and those involved.
The second paragraph entails your personal feelings or reaction to the situation and how it made you feel.
The third paragraph can include making sense of the situation. You have to think about why things happened the way they did. You should also critically review and develop insights based on the situation. Finally, think of the factors that could have influenced the situation.
The next paragraph should explain how the event or situation will change your practice, approach, decisions, perspective, or perception. This is where you evaluate the experience by detailing the knowledge and skills you took from the experience.
The last body paragraph should entail a critical reflection on the learning opportunities. First, describe the situation and what it made you learn. Next, elaborate on how you intend to make yourself better poised to address such situations.
Mostly, you should structure the body of your essay as per the preferred nursing reflective model.
After everything else falls into place, you need to summarize the information you presented in the essay. Then, finally, restate your thesis and have a call to action to bring a sense of closure to your readers.
Steps for Writing a Nursing Reflection Essay � The Guide
When assigned to write a reflective essay for your nursing class, here are the surefire steps to get you to success.
Read the instructions
The first step after receiving an assignment is to begin reading the instructions. as you read, note what your instructor or professor expects in the paper you will submit for marking.
Reading instructions helps you to get informed on the scope of the paper, word count, number of references and pages, and the formatting style to use.
Besides, you also get to plan your paper with the deadline highlighted in the instructions.
You need to get a conducive environment where you can start writing.
The first step of writing is to brainstorm about situations during your clinical hours when you were shadowing a Nurse Practitioner or one you have read about.
Assess whether the situation or scenario you have thought, encountered, or chosen can help you write a reflective essay that meets the requirements.
Research and Plan
After choosing a scenario, the next step is researching the best reflective model.
You can use your class text, the instructions, the college library, course readings, and online nursing journals to get articles and resources with information about specific reflective models.
Select the best reflective model and take notes on the steps it entails.
As you research, write down notes on how to address your paper based on your selected framework or model of reflection . Additionally, research nursing journal articles with information you can use when critically analyzing a situation.
Plan how you will handle the paper as well. For instance, as you research, develop a thesis statement that grounds your entire paper, then draft an outline on how to develop the thesis.
Write an Outline
Outlining is a crucial aspect of writing. It helps you envision how you will meet the objective of writing a reflective essay. As an essential part of the essay writing process, outlining helps create a good flow of ideas and can come in handy in helping you overcome writer's block. Your outline should comprise the following:
- The hook or attention grabber
- Thesis statement
- Main points of each body paragraph (topic sentence, evidence, examples, illustrations, etc.)
- Conclusion (restated thesis and call-to-action)
With the outline done, you should take a break and resume writing your first draft of the nursing reflection essay. Writing with an outline helps avoid mistakes and also helps you write faster.
Describe the Experience
Once you have identified the relevant experience, begin describing it chronologically.
Describe the experience that prompted you to consider nursing your ideal career goal. Think of this experience's key elements, such as the setting, patient demographics, and significant events that impacted you.
Show how these events changed your perspective on life. Ensure you are as descriptive as possible to paint a clear picture for readers.
Consider the following questions to come up with a good description:
- What happened?
- Was there someone involved? If yes, what part did they play?
- Where did the event take place?
- What actions did you take?
Set the context of this experience by giving relevant background information. Ensure you are objective and pay attention to the facts.
Provide a Reflection
Talk about your feelings and thoughts concerning the particular experience you went through. You have to be honest and open up about your initial expectations and challenges you faced at each stage of the experience. The following questions can help you come up with a good reflection:
- What was I trying to achieve?
- What prompted me to act the way I did?
- Are there any consequences for my actions? If yes, what are they?
- How did I feel about this event as it was happening?
- How did those around me react to it?
- How do I know how those around me felt about it?
Analyze the Experience
Description of an experience is essential, but so is analysis. You have to move beyond the surface and give a critical analysis of your experience.
State your actions, and your overall experience will give insights into your experience. Think of how the experience has impacted your actions, feelings, and thoughts.
Give an Evaluation
Evaluate the skills and knowledge you got from the experience. Show how you can apply these skills and knowledge in your nursing practice. Also, state the actions and interventions you took during the nursing experience.
State whether you achieved the desired outcome and if there are any specific areas that you need to improve on.
Talk about how you built or improved skills like communication, teamwork, and critical thinking.
As you evaluate the experience, identify what you believe to be your strengths and weaknesses in the nursing experience. What have you learned from the experience? State the areas where you excelled and what abilities contributed to your success.
Talk about how those you were with during the clinical experience complimented you. Similarly, acknowledge your weaknesses.
What kind of mistakes did you make, and how did you improve them? Talk about the tasks that drain you most during the experience.
Illustrate Learning
Demonstrate elements of deeper thought and reflection levels. This is a great point to include nursing theories in your reflection essay to support analysis of your experience.
Relate your experiences to the theoretical frameworks you were taught in class. This is effective learning and will demonstrate your ability to apply knowledge to real-life nursing situations.
Doing this will also show that you can effectively deduce different things from observations made during the reflection process.
Ensure you also demonstrate a change in perspective, as this will prove that you learned something from the experience.
Write Your Conclusion
Conclude by summarizing your points and highlighting the lessons learned.
The lessons you reached as part of your reflection should support your overall conclusion.
Also, restate your thesis statement.
Come Up with an Action Plan
Now that you have learned from your reflection develop an action plan for future nursing practice.
This part should contain all the details you have learned and actions needed to improve when faced with a similar situation. Consider the following questions:
- What would I change if faced with a similar situation?
- How can I develop the necessary skills needed to face this situation?
- How can I act differently in a similar situation?
Ensure you identify areas to improve and set realistic goals to enhance your nursing skills. Discuss how you intend to seek additional education, training, or mentorship to address your shortcomings.
Finally, end the essay with a happy note so readers know you learned something from the experiences.
Proofread, Edit, and Polish
After doing your first draft, take a break to relax and get out of the writing mood - it helps you to become objective.
You can then resume reading out loud to yourself, make necessary tweaks, and ensure that every part you include meets the rubric requirements.
Edit for grammar, punctuation, tenses, voice, spelling, and use of language. You should also proofread the essay to adhere to the style, organization, and presentation requirements.
Ensure that all the in-text citations are accounted for in the reference list and are up-to-date. You are good to go when you have an essay that meets all the instructions.
Finally, you can submit the paper for grading.
Writing is not everyone's cup of tea. For that reason, you can hire a nursing reflection essay writer from our website to assist you in crafting a top-grade paper. In addition, we have nursing writers whose forte is writing various nursing papers.
Choosing the suitable Reflective Model or Framework
As you can see above, many reflective models are used for your reflective essay. We have not exhaustively listed and expounded on all of them. Other reflective models and frameworks you can also consider when writing a reflective essay in nursing include:
- Bouds Reflective Model
- Brookfield Reflective Model
- Pender's Health Promotion Model
- Roper Logan and Tierney Model
- Driscoll Reflective Model
- The Johari window model
Note that most nursing instructors will often suggest the models they prefer for you to use in your essay.
For example, in most nursing reflective essays. Whichever the case, readily available information expands on each model to make it easier to write a reflection essay on a specific aspect of nursing education or practice.
Read the assignment rubric and instructions to understand the specific model. If it is unclear, ask for clarification from your instructor early enough.
Tips for Writing a Good Nursing Reflective Essay
As you try to figure out how to write a nursing reflective essay, keep the following tips in mind.
Choose the Right Topic
If the instructions from your professors involve choosing a topic for the reflective essay, you must select one that is meaningful to you.
This will ensure you can easily write and easily develop relevant elements about the topic. Therefore, take time to pick a topic that you find interesting.
As you write, ensure you stay on topic, whether sharing a one-off event or a recurrent story.
Use the Right Tone
A reflective essay is more personal, unlike other types of academic essays. This means you don't need a strict or formal tone.
Since this is about your experiences, use personal pronouns such as I and Me.
Be Vulnerable
You must be extremely vulnerable to learn how to write a reflective essay in nursing.
Be open about your thoughts, feelings, and beliefs about something you went through that sparked an interest in nursing.
It's okay to share mistakes or things you did wrong that eventually led you to this career path.
Choose the Right Focus
A reflection essay is all about narrating your experience during the nursing experience.
While including other people in your experience is okay, please let them not be the center of your reflection.
This is your essay, so you should be the focus of attention.
Keep it Brief
A good nursing reflection essay should be between 300 and 800 consciously written words. Because of this length, you must only write relevant information about your reflection. Refrain from lengthy reflections, as they make it difficult to pass your points across.
Convey Your Information Wisely
Even though a nursing reflection essay is about your personal experiences, it doesn't mean you should reveal everything about yourself. Ask yourself whether something is appropriate before including it in your paper.
Mistakes to Avoid When Writing a Reflection Essay in Nursing
A good reflection essay involves reflecting on your nursing studies and practices throughout school and career to demonstrate your competence. For this reason, there are certain mistakes you should be aware of when writing an essay.
Not including a Personal Story
Like food tastes bland without salt, so does a reflection essay without a personal story. At the center of a reflection essay is You. This means the essay should focus on your personal story that led you to want a nursing career. A lot of times, students miss out on this instead of talking about their story. You need more than just the personal qualities you think will be a great fit for the nursing program; you must also share a story that shows how well you contributed to nursing care.
Failing to Share Your Experience
You will lose points when you fail to include nursing-related experiences in your reflection essay. Mentioning that you want to be a nurse is great, but failing to show specific events that led to the desire will cost you a great point.
Plagiarizing Your Essay
Plagiarism is a serious academic offense because it is considered taking other people's ideas and using them as your own without crediting the author. So, provide relevant citations and references for any ideas that aren't your own. Also, an AI will not write your essay as a human writer would.
Related Readings:
- How to write a student nurse resume with no experience.
- PICOT examples and guides
Sample of a Nursing Reflective Essay
The following is a sample of a nursing Reflective essay using Gibb's Model of Reflection. Use this sample to guide you when writing your own.
Introduction Communication is an important element in healthcare practice as it determines patient satisfaction and treatment outcomes. This essay will focus on reflecting on an experience I went through with a 40-year-old diabetic patient who also had a foot infected with an ulcer. When I approached the patient to sign the consent form, I noticed that he wasn't happy because of the news given to him about his health. I concluded that there must be a communication dilemma. I will reflect on the experience using Gibb's Model of Reflection. Using this model, I will identify and discuss the actions taken to resolve the issue. Description This incident happened a few months ago when I was working as a wound nurse in a Methodist hospital in my hometown. I was part of a care team handling the case of a 40-year-old male patient with diabetes and an infected diabetic foot ulcer. After careful examination, a team comprising various specialists concluded that his leg needed amputation below the knee. After making this decision, the team left, and I was asked to give the patient a consent form to sign. When I came back from retrieving the form, I noticed the patient looked sorrowful because of the news given to him. Feelings As soon as I saw the patient, I knew what he was going through. He perceived the situation to be irreparable, but I wasn't sure whether to console the patient or not. I was powerless and couldn't imagine what he was going through. At the same time, I was startled that the team left without showing any compassion. They could have handled the situation more delicately. I, on the other hand, could have relayed the information better. I wasn't sure whether my approach would be acceptable or appropriate. Evaluation I always go back to that particular situation and wonder whether I could have acted better. The situation helped me better understand the importance of good communication in patient care, particularly in therapeutic care. Before the incident, I didn't acknowledge the role of nurses play in caring for patient's emotional needs. I realized nurses must show compassion and console patients in their low moments. Analysis Most healthcare professionals do not know how to deliver bad news to patients. They find the process extremely challenging and always feel psychologically unprepared. This has a negative impact on patients and could lead to bad health outcomes. Furthermore, how information is relayed could impact a patient's adherence to treatment. Because of these effects, multiple protocols and approaches were developed to help with communicating bad news to patients. One of the approaches that was proposed is emotion-centered. This proposes that a healthcare provider acknowledges how sad the patient is and builds a professional relationship based on empathy and sympathy. Action Plan I now understand the essence of communicating bad news with compassion. The experience allowed me to look closely at different aspects of my professional development that needed more improvement. Thus, I plan to be more empathetic and speak up in support of patient's emotional and psychological well-being, especially when presented with traumatic news about their health. Additionally, I now understand I am not powerless when dealing with a sorrowful patient. I believe I have learned from my experience, and I'm not able to communicate well with patients any more. Conclusion The experience allowed me to value good communication in nursing and the need to incorporate it into daily nurse-patient interaction. Nurses must learn how to deliver bad news and manage patient's sorrow. This has been and will continue to be my biggest priority in patient care. References Street Jr, R. L., Makoul, G., Arora, N. K., & Epstein, R. M. (2009). How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient education and counselling, 74(3), 295-301. Buckman, R. (1992). Breaking bad news: why is it still so difficult? BMJ: British Medical Journal, 304(6842), 886. Ptacek, J. T., & Eberhardt, T. L. (1996). Breaking bad news: a review of the literature. The Journal of the American Medical Association, 276(6), 496-502.
Writing is not everyone's cup of tea. For that reason, you can hire a nursing reflection essay writer from NurseMyGrade to assist you in crafting a top-grade paper. In addition, we have nursing writers whose forte is writing various nursing papers. Just place an order , and we will get back to you ASAP.
Struggling with
Related Articles

iHuman Tips and Tricks for Success
The Best Online Nursing Schools and Programs

The Fraudulent Nursing Diploma Scheme
NurseMyGrades is being relied upon by thousands of students worldwide to ace their nursing studies. We offer high quality sample papers that help students in their revision as well as helping them remain abreast of what is expected of them.
Nursing Reflective Essay: Examples + Useful Writing Tips [2024]
![nurse reflective essay examples Nursing Reflective Essay: Examples + Useful Writing Tips [2024]](https://studycorgi.com/wp-content/uploads/2022/04/medical-assistant-reading-book-elder-disabled-woman-nursing-home-senior-patient-sitting-wheelchair-receiving-assistance-support-from-nurse-as-entertainment-fun-activity-485x528.jpg)
Do you need to write a reflective nursing essay? Whether you are a nursing student or already working, we know that you may be too busy writing papers. We’ve prepared information for you about reflection models and nursing reflective essay examples, writing instructions, and templates for the outline. This article will teach everything you need to make writing your essay easier.
So, how do you write an academic reflective essay?
What Is a Reflective Essay?
15 best reflective essay topics: nursing, nursing reflective essay examples, reflective tools and models, reflective essay writing tips, reflective essay body paragraphs, how to conclude a reflective essay.
In a reflective essay, you express your thoughts based on your knowledge, beliefs, reflections, and experiences. A reflective essay is best described as an exceptional and personal essay: you will have a lot to think about, comprehend, and explain. You have to demonstrate the feelings that you have experienced before. It should be honest. If you have any opposing thoughts and feelings about the topic, you should write them.
| Criteria | Reflective essay | Analytical essay |
|---|---|---|
| Subject | Reflection on previous experiences | Analysis of a controversial topic |
| Writing style | Free and relaxed | Formal |
| Object | Personal experience | Depends on subject |
| Thesis statement | Focus on the narrative | Focus on the paper’s topic |
The peculiarity of a reflective essay, unlike an analytical one, is in the expression of thoughts based on personal experience.
How long does a reflective essay last? It’s simple. It depends on your topic and how you express your thoughts. But you have to remember that this is a highly brief type of essay, and you can do it with a few pages.
You can check out the 100% free essay samples to see it all.

What Is a Reflection in Nursing Essay?
Nursing involves many aspects of work, and reflection is one of the critical tools for developing professional competence and personal skills. In nursing, reflection is important because it provides all the prerequisites for further personal growth. It is a reflection that will allow you to take a broader look at your strengths and weaknesses and assess your prospects.
Think of your nursing reflective essay as a chance to reflect on your career, skills, and personality, which will lead to further improvement.
- Empathy and support in nursing.
- Communication with patients is one of a nurse’s most important tasks.
- Nursing is the art of caring for the patient during illness.
- The key is not to cause harm but to help afterward.
- Time management in nursing.
- The Patient’s Interests Come First.
- Why did I become a nurse?
- Doctor- patient confidentiality in nursing.
- The importance of enhancing soft skills for the nurse.
- Nursing knowledge should not be limited to narrowly professional information.
- Reflective essay on the internship .
- Nursing culture and ethics .
- Deontology of nursing and its synthesis with my personal experience.
- Nursing clinical reflection essay.
- Nursing issues for the elderly.
To better understand what reflexivity is, let’s look at examples based on these articles:
| The author considered the best competencies that fit her current and future goals. She discusses the skills acquired and draws conclusions. | |
| The author takes a reflection on the DNP course. Conclusions about specific skills support it. | |
| The author reflects on DNP Essentials, which is the author’s course. Reflection occurs in terms of its contribution to the author’s professional development. | |
| An example of the subject of leadership. The author took a course on reflective practice in nursing and discovered many new things. | |
| In this essay, the author reflects on her experiences and skills in the context of Capstone’s professional practice. The reflection occurs about oneself and concerning others involved in the practice. | |
| The author discusses the skills he learned while creating and launching his course for nurses. | |
| The author reflects on the experiences nurses have had due to taking his course and what is vital in nursing. | |
| The author reflects on her personal experience with the patient. Great for students who already have experience in the specialty. | |
| The author discusses the challenges nurses face and the ways to solve them. | |
| In this paper, the author discusses the goals, strategies, and other aspects of implementing artificial intelligence in nursing. | |
| This essay examines nursing philosophy and its relationship with individual values. | |
| In this essay, the author breaks down the benefits and disadvantages of introducing case management into nursing. | |
| The author explains how the Civil War in America changed the role of women in nursing. | |
| The author discusses the issue of bullying and violence in nursing and provides suggestions and reasons. | |
| This paper examines the functions such as hospital premises and toilet cleaning, which initially are not the responsibility of nurses, and other staff is to fulfill them. | |
| This evidence-based project presents data that aims to evaluate the efficiency of team-based anti-stress therapy sessions and compares it with standard self-care practices in a hospital setting. | |
| The author discusses the ways to reduce the pressure on nurses and reflects on personal experience. | |
| This paper breaks down the responsibilities of RNID. | |
| The author explains three strategies to create a well-developed portfolio for nurses. | |
| This short paper aims to discuss specific challenges and risks that nurses experience in work environments. |
Scientists from different fields of science invented many models to simplify the process of reflection. Below are reflection models you can use in your essay nursing writing practice.
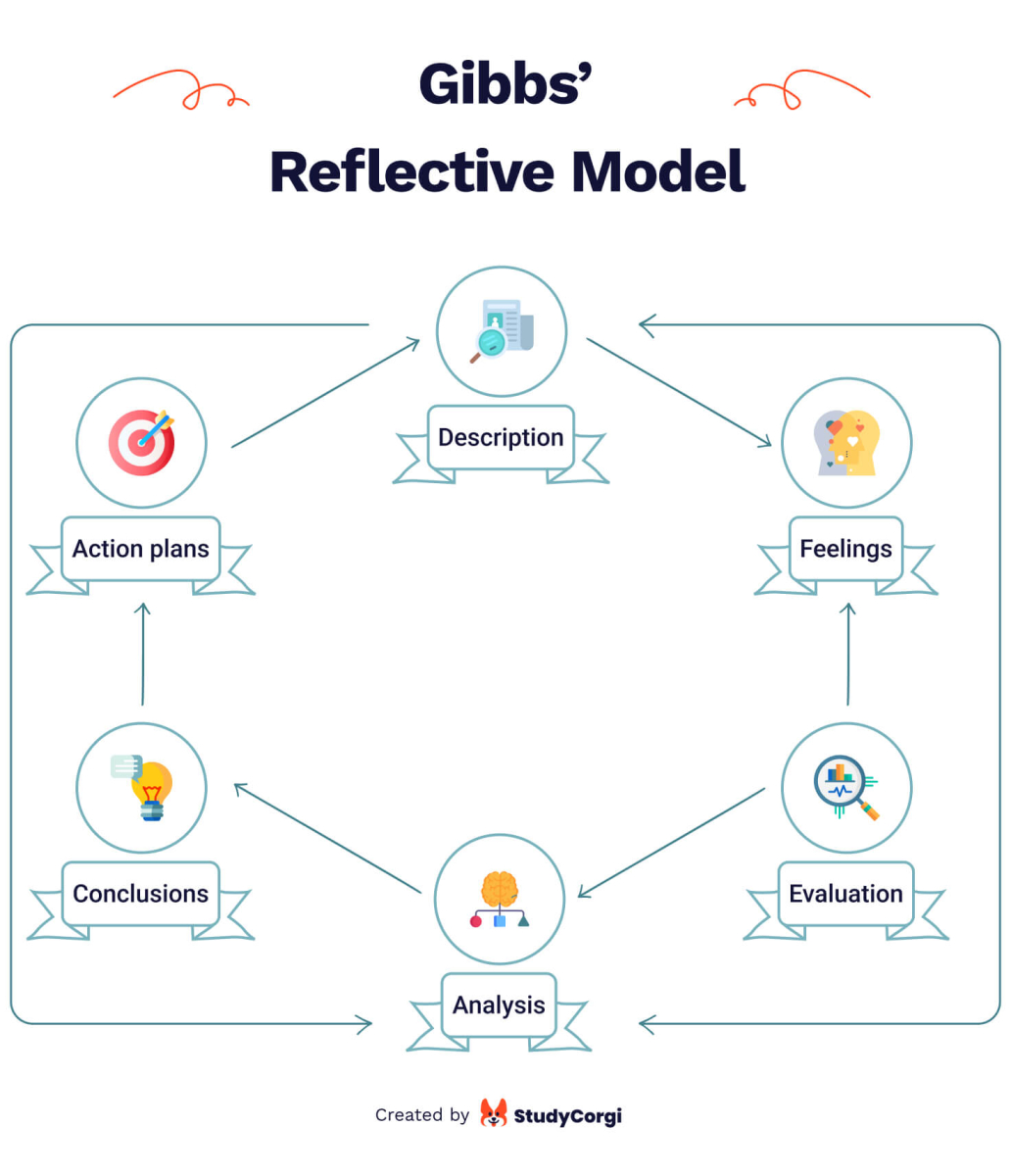
Gibbs’ Reflective Model
In 1998, Graham Gibbs introduced his reflexive model to the world. Gibbs’ model is a cycle and, therefore, excellent for analyzing repetitive experience.
It covers 6 stages:
- Description. What happened? Don’t judge or try to conclude yet; just describe.
- Feelings. What were your reactions and emotions? Again, don’t begin to analyze them yet.
- Evaluation. What was good or bad about the experience? Make value judgments.
- Analysis. Were different people’s experiences similar or different in importance?
- Conclusions. What conclusions can you draw in a general sense from this experience and your analysis? What conclusions can you draw about your particular, unique personal situation or way of working?
- Personal action plans. What steps are you going to take based on what you have learned? What are you going to do differently in this situation next time?
Dewey’s Reflective Thinking Model
John Dewey believed that reflective thinking is the active, persistent, and careful evaluation of a belief or assumed form of knowledge, the grounds for that knowledge, and the additional conclusions to which knowledge leads.
John Dewey’s Reflective Model was one of the first and has been the foundation for many other models. He identified five steps of reflective thinking:
- Step 1: Identify the problem.
- Step 2: Investigate the issue.
- Step 3: Generate several possible solutions.
- Step 4: Evaluate the options and select the best answer from the combination of solutions.
- Step 5: Test and implement the solution.
Kolb Reflective Model
The Kolb Reflective Model or “Kolb cycle” focuses on transforming information into knowledge. The basic four steps of the Kolb model are as follows:
- Concrete experience. Anyone should already have experience in the field or area they want to learn.
- Observation and reflection . This stage implies analyzing the person’s experience and knowledge .
- Forming abstract concepts . A model describing the information and expertise is built at this stage . Ideas are generated, interrelations are made, and new information is added concerning how everything works and is arranged.
- Testing in new situations . The last stage implies experimentation and testing the model’s applicability and concept . The result of this stage is a direct unique experience. Then the circle closes.
Schön Reflective Model
Donald Schön’s reflexive model was described in the book ‘ The Reflexive Practitioner .’ He explained how professionals solve problems with a kind of improvisation that is perfected through practice.
Schön’s reflective model , like his writings, is based in many ways on the Dewey we already know
- Action reflection involves reflecting on an experience you have already had or an action you have already taken. It involves considering what could have been done differently and the positive aspects of that interaction.
- Reflection in action involves reflecting on your efforts as you make them and considering issues such as best practices throughout the process.
Bouds Reflective Model
David Bouds paired with Schön to explore the limits of reflective practice. This model is based on learning by doing. By analyzing his own experience, the practitioner begins to understand better how to improve certain things.
Bouds suggests that by reflecting, a person may be unconsciously learning. In reassessing the events of his life, he systematizes and classifies emotions, ideas, and results, as well as results, and compares past goals with results.
Past experiences, experiences, and ideas require constant analysis attention to feelings. This leads to new perspectives, commitment to action overall positive changes in behavior.

Driscoll Reflective Model
John Driskoll proposed a simple model of reflection at the beginning of the 21st century. It involves reflection through extended answers to questions we are already familiar with. The scholar linked the three basic questions to the stages of the experiential learning cycle and then added trigger questions that must be answered to complete the process of reflection.
Step 1: What? Sets out to recall what happened as objectively as possible, without criticizing anything that happened
- What exactly happened?
- What exactly did you do?
- Was anyone else involved?
- Was it a good experience? Or a bad experience? Or both? And why?
Step 2: So what? It requires you to slow down and start looking for patterns or meaningful moments. The key here is to bring in concepts that help shed light on what is going on.
- How did you feel at that particular moment?
- How did you react?
- Why did you react that way?
- Did you feel the same way about the situation then as you do now?
- Did you experience conflict with your values?
- Do you think past cases influenced your experiences in this situation?
- Who else was involved in the situation? How did they feel? And how did they react? And why did they react that way?
Step 3: Now what? Encourages beginning to transfer new knowledge into future situations and other contexts.
- What did you learn from reflecting on this situation/experience/incident?
- Could you have prevented the negative consequences?
- And how might you have done so?
- What would you have done differently if a similar situation arose in the future?
- What could you have done to better prepare for it?
- Where did things go wrong last time, and what would you focus on now?
5R Framework
The 5R Framework was developed in 2002 by a group of scientists as a universal system of reflection, later modified.
It focuses on five basic steps, each addressing one aspect of reflection. Thinking through the five stages, an individual will engage all of the major reflection components, allowing you to create a critically meaningful review based on your experience.
- Report. What do you see and hear? Write it all down.
- React. What do you think is happening? What works well and what doesn’t? How do you feel about the situation you are reflecting on? What is it about this situation that makes you feel this way?
- Relate. How do my perceptions of this situation relate to my personal and professional experiences? How do they relate to my knowledge and skills? Through what “lens” do you view this situation? From the perspective of classroom management or collaborative learning? Do you need professional development in terms of content knowledge?
- Reason. Do you relate your point of view to someone else’s and how it affects the situation? Does sound practice support your strategies, or do you use different approaches? How does your point of view affect how you understand the problem? Could another point of view be helpful?
- Reconstruct. What did you learn from this observation, and how will it affect your practice?
CARL framework
The CARL framework of reflection involves going through four stages: context, action, results, and learning. The vastness of this model gives a lot of information, and even at the first stage, you can already get a lot of valuable data. But this is also its main disadvantage because it makes the model more complex.
The framework has four steps:
- Context : Description of the context of the experience.
- Action : Explanation of the action taken.
- Results : Explanation of what your actions led to.
- Learning : Identification of the experience and knowledge gained.
Now that we’ve covered the basic concepts let’s write a reflective nursing essay.
Reflective Essay Outline
How to make a reflective essay? First, write an outline.
Any reflective essay is a statement of thoughts about something, and the outline acts as a sketch in which you write them down. Creating it is the first step to creating high quality and vivid essay.
What would make a good outline structure for a reflection essay? Structure all your knowledge. Write what you want to see in the introduction, body, and conclusion.
Here is the reflective essay outline template:
- Hook: Use a catchy statement to set the tone and introduce the article.
- Mention the main points.
- Thesis statement: Include all the important points and ideas in one statement.
- Topic sentence: Carefully explain the first idea in one sentence.
- Proof of topic or idea: Use authoritative sources to support the idea.
- Idea analysis: Apply logic and rational thought to the idea and information from the source.
- Topic sentence: Carefully explain the second idea in one sentence.
- Topic sentence: Carefully explain the third idea in one sentence.
- Repeat the statement of the thesis.
- Review the main ideas
- Final, concluding sentence
Reflective Essay Thesis
A thesis statement is a condensed version of the paper in which you must briefly explain your position. The point is for your audience to read it and understand your work.
To make a good thesis statement for a reflective essay, you need to formulate your thought on paper. To begin with, think well about the problem, and develop your attitude toward it. That’s why the thesis statement should be created after writing the outline. It is unnecessary to give everything out in advance to the reader because later, you will unfold this thought more broadly.
After successfully developing the thesis statement, you can proceed to the actual writing.
And so, below, you can see examples of the reflective essay introduction and conclusion. Learn what its body of it is.
How to Start a Reflective Essay? Examples
You need to start any paper in the right way. How to write a reflective essay introduction? Check these examples:
- Practice and theory do not always agree. Not all of the medical reference books that I have been able to study are fully applicable in practice. John Kimble’s case is a testament that it is often necessary to rely on them and consider the specific situation. He complained of dizziness, and the doctors advised him to get a blood iron test. The test showed that there was nothing wrong. But as it turned out later, this was an erroneous result because Kimble had eaten redfish rich in iron the day before, which temporarily raised the iron in his blood.
- I had always dreamed of becoming a nurse, but my fear of blood robbed me of all desire to study. But still, my passion and ambition made me pull my will into a fist. I had to change and forget what fear of blood was. I lived my dream, which helped me overcome one of my fears.
- A few years ago, I went through a severe emotional crisis. I had always dreamed of going to the University of California, as it is one of the most prestigious medical universities in the United States. But unfortunately, I failed my entrance exams, so I had to study at the university in my hometown. My failure seemed like a disaster, but now I realize it was a real blessing.
The essay’s body carries arguments, explanations of the topic, and the main body of information.
Each paragraph should begin with short introductory sentences, and the body of the sections should be divided into several parts. This will help you better structure what you have written and help the reader navigate through the text.
It is worth remembering about argumentation. There should be several things that you rely on in your position. Describe each of them in detail in a separate paragraph. Observe the semantic sequence. A mistake, in this case, will be to repeat twice the same argument in different words. It will give the reader the impression that there is nothing to say.
You should also remember about transitions in a reflective essay. Move smoothly from one aspect to another and take your time.
How to end a reflective essay? In conclusion, you should focus on pulling together all of the material, summarizing all of the points made and what you have learned. Try to include a few moments about why and how your attitudes and behaviors changed. Here are some examples:
- Was the practice of volunteer nursing helpful to me? Absolutely! I was grateful for these weeks because I learned a lot. But most of all, I have learned my Soft skills, which will help me get used to my professional activities.
- At that moment, I thought about who would be the priority in that situation, and I still believe it was the patient. If the same problem were to arise again, I think I would do nothing but speak up and justify my decisions. I recognize and know that time management and prioritization are essential skills in nursing, and I hope that my skills will improve with practice.
- I reflected on how personal values can affect professional practice from the incident. Still, it is crucial to consider that the organization has its own set of values to follow, regardless of personal values. Finally, in reflecting on the incident, I was able to discuss the importance of quality assurance monitoring and health care evaluation both in general and during the incident, which allowed me to consider what conclusions were drawn from the incident and how to prevent similar situations occurring again.
So, now you have examples and an idea for writing a reflective nursing essay. Writing your paper will become many times easier and faster.
If this article was helpful, share it with your friends and colleagues!
❓ What Is the Purpose of Writing a Reflective Essay?
A reflective essay aims to express one’s thoughts about a previous experience, including how one changed in the process and what one learned. It is often described in a diary entry; they are intended to demonstrate how the author’s thoughts have changed over time.
❓ What Makes a Good Reflective Essay?
A reflective essay is a combination of both objective and subjective elements. We mix scholarly analysis with personal experiences. This type of paper should explain to readers how our experience influences our behavior and what lessons we learned.
❓ What Are the 3 Basic Parts of Reflection Paper?
An essay should have a clear structure and must contain three parts. It should have a clear introduction reflecting the problem, then the central part with an analysis of the causes, and the conclusion with possible solutions to the problem or how it affected the author.
❓ What Is the Best Reflective Model to Use in Nursing?
One of the best options for nurses is the Gibbs model. The model is accessible and extremely simple. Therefore it can be operated without any problems in any situation.
- Reflection toolkit: The University of Edinburgh
- Essay Conclusions: University of Maryland
- Thesis Statements: UNC Writing Center
- A short guide to reflective writing: University of Birmingham
- Outlining: Harvard College Writing Center
- Reflective Practice in Nursing: ZU
- Critical self-reflection for nurse education: NCBI
- Essay Structure: Harvard College Writing Center
- Essay Structure: UAGC Writing Center
- General tips for academic reflections: The University Of Edinburgh
- Reflective essays – ANU
- Writing An Essay Title – Illinois Valley Community College
- Nursing Essay Help: Medical Essay Writing Service in UK
- Share to Facebook
- Share to Twitter X
- Share to LinkedIn
You might also like
30 google search tips & tricks for students, learning to write shorthand: the complete guide for students, infographics for students: the complete guide.

How to Write a Nursing Reflective Essay as a BSN Nursing Student
- November 29, 2023
Welcome to NursingWriters.net, your go-to resource for expert information and guidance on writing nursing essays. In this article, we will provide you, as a BSN nursing student , with a comprehensive guide on how to write a compelling nursing reflective essay. Reflective essays are a personal reflection on your experiences in the nursing profession, and we are here to help you navigate this writing challenge.
As a busy nursing student, we understand that you may have limited time to spare. That’s where NursingWriters.net comes in, empowering you to excel in your BSN program by providing expert guidance on different writing and comprehension challenges you may face. Let’s dive into the world of nursing reflective essays and discover how you can effectively express your thoughts and insights.
Key Takeaways:
- Reflective essays allow nursing students to reflect on their experiences and personal growth in the profession.
- A nursing reflective essay is different from a personal statement, focusing on self-reflection rather than academic achievements.
- Key elements to include in a nursing reflective essay are the inciting incident, personal reflections, vivid details, and actions taken.
- The introduction should grab the reader’s attention and provide a clear thesis statement.
- The body paragraphs should delve into the writer’s reflections and emotions with specific examples and anecdotes.
What Is a Reflective Essay in Nursing?
A reflective essay in nursing is a powerful tool that allows nursing students to analyze their experiences, emotions, and actions related to their nursing practice. It provides an opportunity for self-reflection, critical thinking, and personal growth. Reflective writing can help nurses develop a deeper understanding of their own practice, improve patient care, and enhance their professional development.
Reflective essays in nursing differ from personal statements in that they focus on specific experiences and their impact on the writer’s growth and development. These essays require the writer to critically reflect on their actions, emotions, and thoughts, and identify ways to improve their practice. By examining their experiences and applying reflective frameworks, nursing students can gain valuable insights into their strengths, weaknesses, and areas for growth.
When writing a reflective essay in nursing , it is important to follow a structured approach. This includes describing the incident or experience, analyzing personal thoughts and feelings, exploring the actions taken, and reflecting on the outcomes and implications. By structuring the essay effectively, nursing students can communicate their reflections in a clear and organized manner.
| Key Takeaways: |
|---|
| – Reflective essays in nursing allow nursing students to analyze their experiences and emotions in relation to their practice. |
| – These essays focus on specific incidents or experiences and their impact on the writer’s growth and development. |
| – When writing a , it is important to follow a structured approach to effectively communicate reflections. |
Key Elements to Include in a Nursing Reflective Essay
When writing a nursing reflective essay, it is important to include key elements that will make your essay comprehensive and impactful. These elements will help you convey your thoughts and experiences clearly, allowing the reader to gain a deeper understanding of your reflections on nursing practice.
Inciting Incident or Event
The first key element to include in your nursing reflective essay is the inciting incident or event. This is the moment or experience that triggered your reflection and made a significant impact on your practice. It could be a challenging patient encounter, an ethical dilemma, or a personal realization. By describing this event in detail, you set the stage for your reflective journey.
Personal Reflections
Your nursing reflective essay should also include personal reflections on the experience. This is where you delve into your thoughts, feelings, and emotions related to the inciting incident. Reflect on how the event made you feel, what you learned from it, and how it has influenced your growth as a nursing professional. Be honest and vulnerable in your reflections, as this will allow the reader to connect with your experience on a deeper level.
Vivid Setting and Descriptive Details
To create a vivid and engaging narrative, include specific details that paint a picture of the setting and the people involved. Describe the physical environment, the interactions between healthcare professionals and patients, and any other relevant details that contribute to the overall context of the experience. This will help the reader visualize the situation and understand the complexities of the event.
Actions Taken
Finally, it is important to describe the actions you took in response to the inciting incident. Discuss how you applied your nursing knowledge and skills to address the challenges or opportunities presented by the event. Reflect on the effectiveness of your actions and whether there were any areas for improvement. This demonstrates your ability to critically analyze your own practice and make informed decisions.
By including these key elements in your nursing reflective essay, you can create a comprehensive and impactful piece of writing that showcases your growth and development as a nursing professional.
How to Write the Introduction of a Nursing Reflective Essay
The introduction of a nursing reflective essay plays a crucial role in capturing the reader’s attention and setting the tone for the entire essay. It should provide a concise overview of the main points that will be discussed and create a sense of curiosity and engagement. Here are some tips to help you write an effective introduction for your nursing reflective essay:
- Start with an intriguing opening line: Begin your introduction with a captivating statement or anecdote that relates to the topic of your essay. This will grab the reader’s attention and make them eager to continue reading.
- Provide context and background information: Give a brief overview of the event or experience that you will be reflecting on in your essay. This will help the reader understand the context and significance of your reflections.
- Present a clear thesis statement: Your thesis statement should clearly state the main purpose of your essay and the specific points or themes that you will be exploring. This will give the reader a preview of what to expect in the body of the essay.
By following these tips, you can craft an introduction that captivates the reader and sets the stage for a compelling nursing reflective essay.
“As I walked into the busy hospital ward on my first day of clinical rotation, I couldn’t help but feel a mix of excitement and nervousness. Little did I know that this experience would become a pivotal moment in my nursing journey, shaping my understanding of empathy, communication, and patient-centered care. In this reflective essay, I will delve into the details of this encounter and explore the personal and professional growth that resulted from it.”
With an attention-grabbing opening, providing context, and presenting a clear thesis statement, your introduction will set the stage for a compelling nursing reflective essay that captures the reader’s attention and lays the foundation for your reflections.
Nursing Reflection Essay Tips and Examples
Writing a nursing reflective essay requires careful consideration and thoughtful analysis. Here are some tips to help you craft an impactful and meaningful reflection essay as a BSN nursing student . Additionally, we will provide examples to illustrate how these tips can be applied.
Tips for Writing a Nursing Reflection Essay:
- Start by choosing a specific experience or event that had a significant impact on your nursing practice. This could be a challenging patient encounter, a critical incident, or a personal realization that transformed your perspective.
- Reflect on the experience and consider how it affected your emotions, thoughts, and actions. What did you learn from the experience? How has it shaped your growth as a nursing professional?
- Focus on the key aspects of the experience that were particularly impactful or meaningful to you. Avoid including unnecessary details or deviating from the main message of your reflection.
- Use specific examples, anecdotes, or patient scenarios to support your reflections. This will make your essay more engaging and relatable to the reader.
- Consider the ethical implications of the experience and reflect on how it has influenced your approach to patient care and decision-making.
- End your essay with a reflection on the implications of your learning for future nursing practice. How will you apply the lessons learned to provide better care and improve patient outcomes?
Now, let’s take a look at two examples of nursing reflective essays to further illustrate these tips:
“During my clinical rotation in the Intensive Care Unit (ICU), I encountered a complex patient case that challenged my critical thinking skills and decision-making abilities. The patient was a middle-aged woman who had undergone a complicated surgery and experienced numerous post-operative complications. This experience taught me the importance of collaboration within the healthcare team and the significance of advocating for the patient’s best interests…”
“One of the most significant experiences during my nursing education was my time spent in the pediatric oncology unit. Witnessing the resilience and bravery of children facing life-threatening illnesses had a profound impact on my perspective as a nurse. It taught me the importance of providing holistic care, not only addressing physical needs but also supporting emotional well-being and promoting a positive environment for healing…”
These examples demonstrate how personal reflections, specific details, and professional insights can be incorporated to create a compelling nursing reflective essay. Remember to structure your essay in a clear and organized manner, ensuring that your reflections flow logically and coherently.
| Key Elements | Example 1 | Example 2 |
|---|---|---|
| Inciting Incident | Complex patient case in the ICU | Experience in pediatric oncology unit |
| Reflections | Importance of collaboration and advocacy | Focusing on holistic care and emotional well-being |
| Specific Details | Complications post-surgery | Resilience of children facing life-threatening illnesses |
| Professional Insights | Importance of critical thinking and decision-making | Promoting positive healing environment |
By following these tips and utilizing examples, you can create a compelling nursing reflective essay that showcases your growth and development as a nursing professional.
How to Conclude a Nursing Reflective Essay
The conclusion of a nursing reflective essay serves as the final reflection on the writer’s growth and learning from the experience. It is an essential part of the essay that summarizes the main points discussed and leaves a lasting impression on the reader. The reflective essay conclusion should bring closure to the essay by restating the thesis statement and highlighting the key takeaways from the essay.
To write a strong and impactful conclusion, start by restating the thesis statement in a clear and concise manner. This reminds the reader of the main focus of the essay and reinforces its significance. Next, summarize the key points discussed in the body paragraphs, highlighting the most important insights and reflections. This helps to reinforce the main ideas and ensures that they are not overlooked in the final reflection.
In addition to summarizing the main points, a thought-provoking statement or future outlook can be included to provide a sense of closure and leave the reader with something to ponder. This can be a reflection on how the experience has influenced the writer’s future practice or a call to action for continued personal and professional growth. By ending the essay on a strong and meaningful note, the conclusion enhances the overall impact of the nursing reflective essay.
A well-crafted conclusion is essential for a nursing reflective essay as it reinforces the main ideas, leaves a lasting impression on the reader, and provides a sense of closure. By following these tips, nursing students can create a powerful and impactful conclusion that enhances the overall effectiveness of their reflective essays.
Tips for Writing a Nursing Reflective Essay
Writing a nursing reflective essay can be a challenging task, but with the right approach, it can also be a rewarding experience. Here are some helpful tips and strategies to guide nursing students in their essay writing process:
- Start early: Give yourself plenty of time to brainstorm ideas, reflect on your experiences, and write and revise your essay. Starting early will help you avoid last-minute stress and allow for a more thoughtful and polished essay.
- Conduct thorough self-reflection: Before you begin writing, take the time to reflect on your experiences and emotions related to your nursing practice. Consider how these experiences have shaped your growth and development as a nurse, and what lessons you have learned along the way.
- Organize your thoughts and ideas: Create an outline or a rough structure for your essay to ensure a logical flow of ideas. Group similar thoughts and reflections together to create cohesive paragraphs, and use headings or subheadings to further organize your essay.
- Seek feedback: Share your essay with trusted peers or instructors and ask for their feedback. They can offer valuable insights, provide constructive criticism, and help you refine your essay to make it stronger and more impactful.
Additionally, it is important to pay attention to the technical aspects of your essay:
- Grammar and spelling: Proofread your essay carefully to ensure it is free of any grammatical or spelling errors. Use grammar and spell-check tools, and consider asking someone else to review your essay for a fresh perspective.
- Coherent writing style: Use clear and concise language to convey your thoughts and reflections. Avoid excessive jargon or technical terms, and focus on communicating your ideas effectively.
- Formatting guidelines: Follow any formatting guidelines provided by your instructor or institution. Pay attention to font style, size, spacing, and citation style if required.
By following these tips and strategies, nursing students can approach their reflective essay writing with confidence and produce compelling and insightful essays that showcase their growth and development in the nursing profession.
Reflective Tools and Models for Nursing Reflective Essays
Reflective tools and models can provide structure and guidance for nursing students when writing reflective essays. These tools help organize thoughts and experiences, allowing for a more comprehensive and meaningful reflection. By utilizing reflection models , nursing students can enhance their reflective writing skills and deepen their understanding of their own growth and development as healthcare professionals.
Gibbs’ Reflective Model
“Reflective practice is both an art and a science that requires ongoing commitment and practice.” – Gibbs
Gibbs’ Reflective Model is a widely used reflection framework in nursing. It consists of six stages: description, feelings, evaluation, analysis, conclusion, and action plan. This model encourages a structured approach to reflection, allowing the writer to systematically explore their thoughts and emotions, analyze the situation, and identify areas for improvement or further development.
Dewey’s Reflective Thinking Model
“We do not learn from experience, we learn from reflecting on experience.” – Dewey
Dewey’s Reflective Thinking Model focuses on the importance of reflection as a tool for learning and growth. It emphasizes the need to actively engage with experiences, thoughts, and emotions, and to critically evaluate them in order to gain deeper insights and understanding. This model encourages nursing students to think analytically and develop a continuous learning mindset.
Kolb Reflective Model
“Knowledge results from the combination of grasping experience and transforming it.” – Kolb
The Kolb Reflective Model is based on the concept of experiential learning. It consists of four stages: concrete experience, reflective observation, abstract conceptualization, and active experimentation. This model encourages nursing students to engage in a cyclical process of learning, where they actively participate in experiences, reflect on them, conceptualize their insights, and apply their learning in real-life situations.
Schön Reflective Model
“Reflection-in-action is the heart of the learning process.” – Schön
Schön Reflective Model emphasizes the importance of reflection in the midst of action. It focuses on the ability to think and adapt in real-time, making decisions based on professional knowledge and experiences. This model encourages nursing students to develop a reflective mindset that allows them to learn and grow while actively engaging in their practice.
| Name | Key Concepts | Stages |
|---|---|---|
| Gibbs’ Reflective Model | Structured reflection, comprehensive analysis | Description, Feelings, Evaluation, Analysis, Conclusion, Action Plan |
| Dewey’s Reflective Thinking Model | Active engagement, critical evaluation | Experience, Reflection, Interpretation, Evaluation |
| Kolb Reflective Model | Experiential learning, application of knowledge | Concrete Experience, Reflective Observation, Abstract Conceptualization, Active Experimentation |
| Schön Reflective Model | Reflection-in-action, adaptability | Reflection-in-Action, Reflection-on-Action |
Benefits of Reflective Writing in Nursing
Reflective writing plays a crucial role in nursing practice, offering numerous benefits for nursing students and professionals alike. By engaging in reflective writing, nurses can enhance their self-awareness, develop their critical thinking skills, and gain a deeper understanding of patient experiences. This section will explore the advantages of reflective writing in nursing and its significance in healthcare.
Enhanced Self-Awareness
Reflective writing fosters self-reflection, allowing nurses to examine their thoughts, emotions, and actions in various clinical situations. Through this process, they gain a deeper understanding of their strengths, weaknesses, and areas for improvement. This heightened self-awareness enables nurses to provide more effective and compassionate patient care, fostering a patient-centered approach.
Improved Critical Thinking
Reflective writing encourages nurses to think critically about their experiences and the impact of their actions. It requires them to analyze and evaluate the effectiveness of their decision-making and problem-solving skills. By reflecting on past experiences, nurses can identify areas where they can enhance their clinical practice and make informed decisions based on evidence and best practices.
Increased Empathy and Understanding
Through reflective writing, nurses develop a deeper empathy and understanding of patient experiences. By reflecting on their interactions with patients, nurses can recognize the emotions, fears, and challenges faced by individuals in their care. This increased empathy enables nurses to provide more holistic and patient-centered care, improving the overall healthcare experience for patients.
Continuous Professional Development
Reflective writing is an essential tool for nurses’ continuous professional development. It allows them to document their growth, learning, and achievements throughout their nursing career. By regularly engaging in reflective writing, nurses can identify areas for further development, set goals for improvement, and ensure they are providing the best possible care to their patients.
Overall, reflective writing in nursing is a powerful tool that empowers nurses to enhance their self-awareness, critical thinking skills, and empathy. By engaging in this practice, nurses can continuously improve their clinical practice, provide high-quality patient care, and contribute to the ongoing development of the nursing profession.
Nursing Reflective Essay Examples
Looking for inspiration for your nursing reflective essay? Here are some examples that showcase different experiences, reflections, and growth in the nursing profession.
| Topic | Key Reflections |
|---|---|
| Providing End-of-Life Care | The writer reflects on their emotions, challenges, and personal growth while caring for terminally ill patients. They explore the importance of empathy, communication, and self-care in delivering compassionate end-of-life care. |
| Topic | Key Reflections |
|---|---|
| Leadership in Nursing | The writer reflects on their experiences as a nurse leader, discussing effective leadership strategies, challenges faced, and the impact of their leadership style on the nursing team and patient outcomes. |
| Topic | Key Reflections |
|---|---|
| Dealing with Ethical Dilemmas | The writer reflects on a challenging ethical dilemma encountered in their nursing practice. They explore the ethical principles involved, their decision-making process, and the lessons learned from navigating complex ethical situations. |
These examples demonstrate how nursing reflective essays can provide valuable insights into personal experiences, reflections, and professional growth. They serve as excellent references to help nursing students develop their own reflective writing skills and gain a deeper understanding of the nursing profession.
Writing a Nursing Reflective Essay for Nursing School Application
A nursing reflective essay can be a powerful tool for nursing school applications. It allows applicants to showcase their self-awareness, critical thinking skills, and passion for the nursing profession. When writing a nursing reflective essay for a nursing school application, there are a few key tips to keep in mind.
Reflect on your passion for nursing
Start by reflecting on what drew you to the nursing profession and why you are passionate about it. Consider your personal experiences, such as volunteering or shadowing healthcare professionals, and how they have shaped your desire to become a nurse. Highlight your commitment to providing compassionate and quality patient care.
Share personal experiences in healthcare settings
Provide specific examples of your experiences in healthcare settings, such as clinical rotations or internships. Reflect on the challenges you faced, the lessons you learned, and how those experiences have impacted your growth and development as a future nurse. Discuss any significant interactions with patients, healthcare teams, or mentors that have shaped your understanding of the nursing profession.
Align with the nursing school’s values and mission
Research the nursing school’s values, mission, and educational philosophy. Ensure that your reflective essay aligns with these principles and demonstrates your commitment to the school’s mission. Use the nursing school’s prompts or essay questions as a guide to structure your essay and address the specific criteria they are looking for in applicants.
By following these tips, you can write a compelling nursing reflective essay that effectively conveys your passion for nursing, showcases your experiences, and aligns with the nursing school’s values. Remember to proofread your essay carefully for grammar and spelling errors and seek feedback from mentors or trusted individuals in the nursing profession to ensure your essay is clear, concise, and impactful.
| Nursing Reflective Essay for Nursing School Application Tips |
|---|
| Reflect on your passion for nursing |
| Share personal experiences in healthcare settings |
| Align with the nursing school’s values and mission |
(Table) Tips for Writing a Nursing Reflective Essay for Nursing School Application
Writing a nursing reflective essay can be a transformative experience for BSN nursing students. It allows them to gain valuable insight into their own growth and development as future healthcare professionals. Throughout this comprehensive guide, we have provided expert information and guidance on how to write a compelling nursing reflective essay.
By understanding the purpose of a reflective essay and the key elements to include, nursing students can effectively showcase their personal experiences and reflections. Incorporating reflection models such as Gibbs’ Reflective Model or Kolb Reflective Model can also provide structure and depth to their essays.
At NursingWriters.net, we are dedicated to empowering busy nurses and providing them with the tools they need to excel in their BSN programs. Whether it’s writing a reflective essay or any other writing challenge, we are here to support and guide nursing students towards success.
What is a nursing reflective essay?
A nursing reflective essay is a personal essay where the writer reflects on their own experiences and how those experiences have shaped their growth and development in the nursing profession.
How is a reflective essay in nursing different from a personal statement?
While a reflective essay in nursing focuses on the writer’s personal experiences and reflections, a personal statement is more of a formal document that highlights the writer’s qualifications, achievements, and future goals in the nursing profession.
What are the key elements to include in a nursing reflective essay?
The key elements to include in a nursing reflective essay are the inciting incident or event, personal reflections on the experience, specific details to create a vivid setting, and a description of the actions taken by the writer. It is important to avoid including academic details and excessive focus on emotions.
How should I write the introduction of a nursing reflective essay?
To write an engaging and informative introduction for a nursing reflective essay, you can grab the reader’s attention with an intriguing opening line, provide context and background information, and present a clear thesis statement. Focus on the specific event or experience that will be the main focus of the essay.
How should I structure the body paragraphs of a nursing reflective essay?
The body paragraphs of a nursing reflective essay should have a clear structure. Use the first paragraph to present the thesis statement and provide background information on the event. Use subsequent paragraphs to explore your reflections, emotions, and actions taken. Use specific examples and anecdotes to make the essay more engaging.
How should I conclude a nursing reflective essay?
To write a strong conclusion for a nursing reflective essay, you can summarize the main points discussed in the essay, provide a final reflection on your growth and learning from the experience, restate the thesis statement, and leave the reader with a thought-provoking statement or future outlook.
What are some tips for writing a nursing reflective essay?
Some tips for writing a nursing reflective essay include starting early, conducting thorough self-reflection, organizing your thoughts and ideas, and seeking feedback from peers or instructors. Pay attention to proper grammar and spelling, coherent writing style, and adhere to any formatting guidelines provided.
How can reflective tools and models help with nursing reflective essays?
Reflective tools and models provide structure and guidance for nursing students when writing reflective essays. Models such as Gibbs’ Reflective Model, Dewey’s Reflective Thinking Model, Kolb Reflective Model, and Schön Reflective Model can be used as frameworks for organizing thoughts and experiences in a nursing reflective essay.
What are the benefits of reflective writing in nursing?
Reflective writing in nursing offers benefits such as enhanced self-awareness, improved critical thinking skills, increased empathy and understanding of patient experiences, and continuous professional development. It promotes lifelong learning and helps improve patient care.
Where can I find nursing reflective essay examples?
You can find nursing reflective essay examples that showcase different experiences, reflections, and growth. These examples incorporate personal reflections, specific details, and professional insights into nursing practice. They demonstrate the diversity of topics and experiences that can be explored in nursing reflective essays.
How can I write a nursing reflective essay for nursing school applications?
To write a nursing reflective essay for nursing school applications, reflect on your passion for nursing, personal experiences in healthcare settings, and future goals in the nursing profession. Address specific prompts and align the content with the nursing school’s values and mission.
How can writing a nursing reflective essay benefit BSN nursing students?
Writing a nursing reflective essay allows BSN nursing students to gain insight into their own growth and development as future healthcare professionals. It helps improve critical thinking skills, self-awareness, and understanding of patient experiences. It also promotes continuous professional development.
Have a subject expert Write for You Now
Have a subject expert finish your paper for you, edit my paper for me, have an expert write your dissertation's chapter, table of contents.
- Our Guarantees
- Client Reviews
- Privacy Policy
- Terms and Conditions
Knowledge Base
- All Writing Guides
- Nursing Essay Writing Guides
- Topics Ideas
- Nursing Guides
- Nursing Exemplar Papers
- DNP Nursing Paper Examples
- MSN Nursing Paper Writing
Nursing Writers Service
- Write My Nursing Paper
- Nursing Paper Writers
- Nursing Assignment Writers
- Nursing research paper writers
- Nursing Paper Writing Services
- Online Nursing Papers
- Personal Nursing Philosophy Paper
- Nursing Paper Writing Services reviews
- Nursing School Paper Writing Service
- Nursing Research Paper Writing Service
- Nursing Paper Writing Service
- Research Paper Writing Service nurses
- Paper Writing Service Nursing
- Paper Writing Service Reddit nursing
- Paper Writing Service chamberlain nursing
Writing Tools
- Citation Generator
- Topic Generator
- Thesis Generator
- Sentence Rewriter
- Title Page Generator
- Research Paper Title Generator
Use our resources and guides to write perfect papers. You can use our writing service and order customized sample papers without plagiarism!
Disclaimer
NursingWriters.net helps students cope with college assignments and write papers on various topics. We deal with academic writing, creative writing, and non-word assignments.
All the materials from our website should be used with proper references. All the work should be used per the appropriate policies and applicable laws.
Our samples and other types of content are meant for research and reference purposes only. We are strongly against plagiarism and academic dishonesty.


Writing Reflective Essays for Nursing Students
Reflective writing is essential for nurses as it allows them to analyze their experiences, thoughts, and emotions to improve their practice. A nursing reflective essay requires the writer to reflect on a particular experience and explore how it has impacted their personal and professional growth. In nursing and midwifery, reflective essays are often used for self-reflection and self-improvement.
What Is a Reflective Essay?
A reflective essay is a piece of writing that explores a particular experience or event and reflects on its significance. The purpose of a reflective essay is to encourage the writer to think critically about their experiences and to analyze how those experiences have shaped their personal and professional growth. Unlike other types of essays, such as argumentative or expository essays, reflective essays focus on the writer’s personal thoughts and feelings rather than presenting objective facts or arguments.
Importance of Reflective Writing in Nursing
Reflective practice is an important tool for student nurses as it allows them to reflect on their experiences and learn from them. By engaging in reflective writing, nurses can better understand their practice and identify improvement areas. Reflective writing also helps nurses develop self-awareness, crucial for providing high-quality patient care. By reflecting on their experiences, nurses can identify their strengths and weaknesses and work towards becoming better healthcare professionals.
Reflective Essay Outline
A reflection paper follows a basic structure, which includes an introduction, body paragraphs, and a conclusion. The introduction should provide a brief overview of the experience or event being reflected upon and include a thesis statement stating the essay’s main point or purpose. The body paragraphs should explore the experience in detail, providing specific examples and reflecting on its impact on the writer’s personal and professional growth. The conclusion should summarize the essay’s main points and provide a final reflection on the experience. An effective outline is essential for organizing your thoughts and ensuring your essay flows smoothly. When creating an outline for a reflective essay, it is important to consider the main points you want to address and the order in which you want to present them. You should also think about the specific examples and evidence you will use to support your reflections. By creating a detailed outline, you can ensure that your essay is well-structured and coherent.
20 Best Reflective Essay Topics: Nursing
Choosing a topic for your reflective essay can be challenging, especially in nursing, where there are so many experiences to reflect upon. Here are 20 examples of reflective essay topics for nursing students:
- My First Day on the Ward: Navigating emotions, challenges, and expectations.
- The Patient Who Changed My Perspective on Nursing: A personal transformation story.
- Balancing Empathy and Professionalism: Finding the middle ground.
- Handling Medical Ethical Dilemmas: Personal experiences and lessons learned.
- The Role of Continuous Education in Nursing: Adapting to new medical advancements.
- Coping Mechanisms for Nurse Burnout: Personal strategies and recommendations.
- The Value of Interdisciplinary Teamwork: Collaborating with doctors, therapists, and other healthcare professionals.
- Cultural Sensitivity in Patient Care: Lessons from a diverse patient encounter.
- The Evolution of My Communication Skills: From novice to proficient.
- The Most Challenging Shift I Ever Worked: Insights and personal growth from adversity.
- Incorporating Holistic Care in Modern Medicine: Personal experiences and the broader implications.
- End-of-Life Care: Navigating emotions, ethics, and patient wishes.
- The Role of Family in Patient Recovery: Observations from the Ward.
- From Textbook to Reality: Bridging the gap between academic knowledge and practical application.
- Adapting to Different Nursing Environments: From emergency rooms to pediatric care.
- The Emotional Impact of Pediatric Nursing: The highs and lows.
- The Importance of Self-Care in Nursing: Personal routines and their impact on professional life.
- Addressing Mental Health: My Role as a Nurse: Encounters, challenges, and insights.
- Reflecting on Patient Advocacy: Standing up for patient rights and wishes.
- Learning from Mistakes: An introspective look at errors made and the invaluable lessons derived.
When choosing a topic for your reflective essay, it is important to select an experience that is meaningful to you and has significantly impacted your personal and professional growth. Choosing a topic that allows for self-reflection and analysis is also important. Check out these other nursing reflective essay samples:
- Doctor Of Nursing Practice Essentials Reflection
- Case Management In Nursing: Benefits And Disadvantages
- Doctor Of Nursing Practice (DNP) Course Reflection
- Video Reflection On Interprofessional Collaboration For NURS 4010
- Registered Nurse Course Reflection
- Nursing Values And Philosophy In The Correctional Setting
- Nursing Violence In The Workplace
- Nursing Role: Course Reflection And Personal Assessment
- Nursing Professional Capstone And Practicum Reflection
- Nursing Course Reflection And Future Plans
- Nursing History: Today And Beyond
- Doctor Of Nursing Practice Program In Reflections
- Implementing Artificial Intelligence And Managing Change In Nursing
Nursing Reflective Essay Examples
Reading well-written nursing reflective essay samples can be a helpful way to improve your own writing. Analyzing these essays’ structure, language, and content, you can better understand what makes a strong reflective essay. Here are two examples to guide you:
Example 1: Reflecting on a Challenging Patient Interaction
During one of my regular shifts in the Medical-Surgical ward, I encountered Mrs. Smith, a patient known for her particularly assertive demeanor. I was met with resistance as I entered her room to administer her evening medications. She questioned the necessity of each drug, casting doubt on my professional judgment. Initially, I felt a rush of defensiveness, my immediate instinct being to assert my knowledge and the doctor’s recommendations. But I paused, taking a deep breath, recognizing the importance of patient autonomy and their right to understand their care. Rather than reverting to a purely clinical explanation, I sat beside Mrs. Smith and discussed her concerns. I inquired about the root of her hesitancy, which revealed a recent incident where she had suffered side effects from a prescribed medication at another facility. This incident left her feeling vulnerable and distrustful. By understanding this context, my perspective shifted from viewing her questions as challenges to my competence to seeing them as her way of seeking safety and assurance. Upon reflection, this situation underscored the significance of effective communication in nursing. It reminded me of Schön’s “reflection-in-action” model as I adapted my real-time approach based on Mrs. Smith’s feedback. Considering the workload and time constraints nurses often face, dismissing her concerns would have been easy. However, the brief moment I took to understand her concerns resolved her immediate anxieties and built a foundation of trust for our subsequent interactions. I’ve since carried this experience, ensuring I always prioritize patient communication, even facing challenges. Patients like Mrs. Smith have taught me that behind every question or concern is a story, and by taking the time to listen and understand, I can provide care that is not only clinically effective but also emotionally supportive.
In this reflective essay, the writer reflects on a challenging patient interaction and discusses what they learned. The essay begins with an introduction that provides an overview of the experience and states the essay’s main point. The body paragraphs explore the experience in detail, providing specific examples and reflecting on its impact on the writer’s personal and professional growth. The conclusion summarizes the essay’s main points and provides a final reflection on the experience.
Example 2: Reflecting on End-of-Life Care
In the early days of my nursing career, I had the profound responsibility of caring for Mr. Martinez, an elderly gentleman in the final stages of terminal cancer. Assigned as his primary nurse, I observed him gradually weaken, his once-vivacious spirit dimming each day. One evening, as the sunset painted a golden hue on the horizon, he wished to see it for what he felt might be the last time. The weight of his words struck me profoundly, and I felt the rawness of the impending loss. The hospital protocols were strict about moving critically ill patients, but at that moment, recognizing the gravity of Mr. Martinez’s request, I consulted with the on-duty physician. Together, we decided to make an exception. Assisting him in a wheelchair, we moved to the window, silently watching the sunset. Mr. Martinez shed tears of gratitude, and I found myself profoundly moved, our hands intertwined. This experience illuminated the profound nature of end-of-life care. It’s not just about medical protocols but about honoring the human spirit, its desires, and its need for closure. I recalled Boud’s Reflective Model, which emphasizes integrating feelings into the reflection process. The melancholy, the sense of impending loss, and the gratitude were emotions I had to process at the moment and the days that followed. Reflection upon this experience taught me that end-of-life care transcends clinical routines. It’s about recognizing the sanctity of life and the dignity of death. In our profession, we often get wrapped up in technicalities, but moments like these remind us of the deeper essence of nursing. Being present for Mr. Martinez in his final days reshaped my approach to care, reinforcing that sometimes, the most profound healing we can offer is understanding, presence, and the space for a patient to find peace.
In this reflective essay, the writer reflects on their experience providing end-of-life care and discusses the emotions and challenges they faced. The essay begins with an introduction that sets the stage for the reflection and states the essay’s main point. The body paragraphs explore the experience in detail, providing specific examples and reflecting on its impact on the writer’s personal and professional growth. The conclusion summarizes the essay’s main points and provides a final reflection on the experience. By studying these examples, you can understand how to structure your reflective essay and effectively convey your thoughts and emotions.
Tips for Effective Reflection in Nursing
Engaging in effective self-reflection is essential for improving your nursing practice. Here are some strategies for effective reflection:
- Set aside dedicated time for reflection: Schedule a regular time for reflection in your daily or weekly routine. This will allow you to focus on your experiences and give them the attention they deserve.
- Use a reflective journal: Keep a journal to record your thoughts and reflections regularly. This will help you track your progress and identify patterns or trends in your practice.
- Seek feedback from others: Ask for feedback from colleagues, mentors, or supervisors. Their perspectives can provide valuable insights and help you identify areas for improvement.
- Practice active listening: When interacting with patients, colleagues, or other healthcare professionals, practice active listening. This will help you better understand their perspectives and experiences, which can inform your own reflections.
- Be open to criticism: Be open to receiving constructive criticism and use it as an opportunity for growth. Reflect on the feedback you receive and consider how it can help you improve your practice.
- Engage in self-reflection exercises: Many can help you explore your thoughts and emotions. These exercises can range from simple prompts to more structured activities.
Incorporating these strategies into your practice can enhance your self-reflection skills and improve your nursing practice.
Reflective Tools and Models
Several reflective tools and models are commonly used in nursing to guide the reflective writing process. These tools and models provide a framework for organizing your thoughts and reflections. Here are some examples of reflective tools and models used in nursing:
Dewey’s Reflective Thinking Model
John Dewey, a prominent philosopher and educational theorist, posited reflection as a systematic process. Contrary to merely a passive pondering of thoughts, In Dewey’s reflective thinking model , a reflection is a conscious and intentional act. In his framework, the reflection process begins by recognizing a genuine problem. This recognition then moves to a thorough problem analysis and formulation of potential solutions. After deriving possible solutions, the most plausible one is tested, with the final stage being a reflection on the actions taken.
Kolb Reflective Model (Experiential Learning Cycle)
Originating from the insights of educational theorist David Kolb, this model underscores the pivotal role of experience in the learning process. Kolb’s reflective cycle is dynamic, suggesting we start with a concrete experience. This experience becomes the subject of reflection, from which we draw conclusions and construct theories or concepts. These developed theories are tested in new situations, contributing to new experiences and further reflection. It’s a continuous loop of learning through experience and reflection.
Schön Reflective Model
Donald Schön’s model centers on the professional’s ability to reflect when confronted with unexpected situations. Schön’s keen observation led to the classification of reflection into two types. The first, “Reflection-in-action,” is “thinking on one’s feet.” It encompasses reflection that occurs concurrently with action, allowing real-time modifications of techniques. Conversely, “Reflection-on-action” involves retrospection after an event to glean understanding and knowledge from that experience.
Bouds Reflective Model
David Boud championed a reflection model that encourages profound thinking and seamlessly integrates an individual’s feelings into the reflective process. Boud’s approach starts with revisiting the experience, emphasizing the recall of associated emotions and feelings. The next step requires recognizing and confronting any adverse feelings that could impede learning. By synthesizing feelings and the event, a clearer perspective guides future actions.
Driscoll Reflective Model
Developed by John Driscoll and influenced by Borton’s developmental framework , this model is known for its simplicity and easy recall. Driscoll’s model prompts individuals to objectively describe what transpired, posing, “What?” Following this descriptive phase, significance is sought with the query, “So what?” This analytical step paves the way for the final, future-oriented question: “Now what?” Here, the individual identifies lessons learned and considers how they can be integrated into future endeavors.
Gibbs’ Reflective Model
Graham Gibbs extended upon the foundational work of Kolb to produce a more detailed and structured reflective cycle . Gibbs prompts individuals first to describe their experience. This descriptive phase showcases thoughts and feelings during the event. An evaluation phase then discerns the positive and negative aspects of the experience, leading to an analytical phase that seeks to make sense of the situation. Conclusions are then drawn about potential alternative actions, and an action plan is formulated for future similar scenarios, completing the reflective cycle.
Using each model of reflection can enhance your reflective writing and help you better understand your practice. Their distinctions cater to varied preferences and contexts, ensuring that individuals can find a model that resonates best with their reflection style.
Common Mistakes to Avoid in Nursing Reflective Writing
When engaging in reflective writing, it is important to avoid common mistakes that can undermine the effectiveness of your reflection. Here are some common errors to avoid:
- Superficial reflection: Avoid providing a superficial or surface-level reflection. Instead, strive to explore your thoughts and emotions in depth and consider the underlying factors that influence your practice.
- Lack of critical analysis: Avoid describing your experiences without critically analyzing them. Instead, reflect on the impact of those experiences and consider how they have shaped your personal and professional growth.
- Lack of self-awareness: Avoid acknowledging your strengths and weaknesses. Instead, strive to develop self-awareness and identify areas for improvement.
- Lack of evidence or examples: Avoid making general statements without providing specific examples or evidence to support your reflections. Instead, use concrete examples to illustrate your points and provide evidence for your reflections.
- Lack of action plan: Avoid failing to identify actions for future practice. Instead, consider how you can apply your reflections to improve your nursing practice and develop an action plan.
By avoiding these common mistakes, you can ensure that your reflective writing is effective and meaningful.
Final Thoughts on Reflective Nursing Essays
Reflective essays provide a means for nurses to engage in self-reflection and self-improvement. By reflecting on their experiences, nurses can gain a deeper understanding of their own practice, develop self-awareness, and identify areas for improvement. Reflective writing also helps nurses enhance their critical thinking skills, increase empathy and compassion, and promote professional growth and development. By following the tips and strategies outlined in this article, nurses can effectively reflect and improve their nursing practice.
If you’re feeling the weight of encapsulating your nursing journey accurately, we’re here to ease the burden. Our experienced team of writers understands the intricacies of reflective writing. Don’t miss the opportunity to present a reflective essay that resonates deeply and authentically. Place your order with us today, and let us craft a narrative that does justice to your journey.

Reflective Nursing Essay
Reflective nursing essay generator.

Have you ever written a diary where you keep accounts of anything you want to say? May it be in the form of a reflection. Like say at the end of the day, you had done this and done that. It could be work related or personal. A lot of people often keep or write down a reflection paper not because it is necessary or to be used in school or class, but to be able to reflect on the day’s events and be able to release the tension that they may have hid while going about their day. For nurses, this may seem more common than you think.
These reflective essays that nurses do practically helps the majority of them to see how they settle with issues within their work and how they may be able to assess their work or their patients with it. In a way it also helps them take up a way to write what they please and find a way to reflect on it. In general, it is a helpful tool. But how helpful is it you ask? Why is it important? Head on over to the examples and the definition to see. While you’re there, take a sneak peek at the tips to write a good reflective nursing essay.
8+ Reflective Nursing Essay Examples
1. nursing reflective essay template.

- Google Docs
Size: 187 KB
2. Reflective Nursing Leadership Essay

Size: 405 KB
3. Reflective Nursing Essay Example

Size: 411 KB
4. Reflective Nursing Scholar Essay

Size: 313 KB
5. Simple Reflective Nursing Essay

Size: 128 KB
6. Reflective Nursing Experience Essay

Size: 209 KB
7. Reflective Nursing Organization Essay

Size: 103 KB
8. Reflective Nursing Process Essay

Size: 43 KB
9. Reflective Nursing Student Essay
Size: 38 KB

What Is a Reflective Nursing Essay?
To start off, we know what a reflection essay is or what a reflective essay is. We know that a reflective essay is a kind of essay that a person writes to reflect on either a topic they are told to write it for or to reflect about either their work, their school or their personal life. In this case, a reflective nursing essay works the same except this is mainly for how nurses are able to reflect on their job , and to be able to use it as practice for their careers. The purpose of a reflective nursing essay is to be able to let nurses be comfortable with their work. To have a good chance to understand and to explore who they are and what they do. They are also able to write about their patients and how they may reflect on that experience. In addition to that, writing a reflective nursing essay gives them good practice from their course and work. Of course when you write your reflective nursing essay, there are also things you need to consider. Watching how you write and what you write would be the most important thing to consider.
How to Write a Reflective Nursing Essay?
Are you planning on writing a reflective nursing essay? Either for practice or for work. As we know that making reflective essays are necessary, they can also be a bit of a challenge especially if you have no idea where and how to begin. However, there is a solution for that. Just as with any problem, there is always a solution and this one is no different.
1. Begin With a Simple but Concise Introduction
The first tip we have is to begin your introduction with a simple but concise paragraph. This is the beginning of your reflective essay. This is the first thing your readers are keen on knowing what you are going to be writing about. Make sure that you do not divulge too much information nor the lack of it. Simple introductions are there to keep your readers’ curiosity.
2. Make It Personal for Your Readers
Another important thing to remember is that when you write your reflective nursing essay, make sure to make it personal. This is one way of getting your readers to sympathize or to empathize with you. The more personal you are to reflecting about your work, patient or personal life, the more your readers are able to understand you.
3. Reflect on What You Have Gone Through
Aside from making it personal, give yourself a short reflection on what you have learned and gone through. From your experiences at work, to your experience with a patient or even from your personal life. Reflect and write them down. Reflect what you have learned and write about it.
4. Assess and Evaluate Your Reflection
In addition to the three tips, it is also good to try and assess and evaluate what you think of your reflection. This means that do you know how far you have gone through, do you understand why you felt this way. Basically comparing your past notes to help you with your full assessment on your reflection.
5. End Your Essay With a Happy Note
Lastly, end your essay on a happy note. In a way that your readers would know that regardless of how you may have felt, you learned something worthwhile. Hence it is best to end on a happy note. But if your reflection may cause you some hurt, end it the same way but with a positive and hopeful mindset.
What is a reflective nursing essay?
A kind of essay that is used by nurses to reflect on the events that may trigger something from them. This essay helps them in a way they are able to understand and be able to write it down and reflect on it.
How long is a reflective nursing essay?
A reflective essay could be as written a page long or as short as three paragraphs. Depending on the one who is writing it.
How helpful is a reflective essay to nurses?
A reflective nurse essay is helpful as it helps them practice taking notes. Either for their patience, for themselves or about their work. Having this as practice not only helps them, but it also gives them the opportunity to open up from their surroundings by reflecting and observing.
It goes without saying, writing reflection essays do help. A nurse’s job is not considered the easiest nor is it considered the sweetest job in the world. They have to go through a lot of emotional turmoil and a lot of emotional stress. A reflection nursing essay is simply a right tool for them to let it all out and still be able to reflect on what they have learned through their days or through the moments of work.
Text prompt
- Instructive
- Professional
Write a Reflective Nursing Essay on a significant patient care experience.
Discuss the challenges of adapting to rapidly changing health care environments in your Reflective Nursing Essay.
- Nursing School
How to Write “What Nursing Means to Me” Reflective Essay

The “what nursing means to me” reflective essay is something often asked of students entering an accelerated nursing program, a master’s level course for nursing, or some other specialized program. Unlike a nursing school letter of intent or nursing school application cover letter, the “what nursing means to me” reflective essay gives you a chance to state and defend an argument regarding something you feel deeply about, namely, being a nurse. It can detail professional setbacks or successes. It can be a venue to express opinions and criticism of the profession. You can also write about important personal events in the past, before you became a nurse, which had some impact on why you chose this profession. This article will explore the nature of the reflective essay, its essential elements, the structure of an excellent reflective essay and provide examples.
>> Want us to help you get accepted? Schedule a free initial consultation here <<
Article Contents 11 min read
What is a reflective essay.
A reflective essay is like a regular essay, but the subject matter is you. You must state a thesis about yourself and choose a specific event from your past or something similar on which to build an argument supporting your thesis. The main impetus of a reflective essay is to get you to reveal growth and how a particular event in your life changed you, your perceptions, beliefs, and so on.
But there are other things a reflective essay can reveal about you. In the context of writing a reflective essay connected with a nursing school application resume, you can also use the essay to state your larger career goals and ambitions as a nurse and how you see the role changing and evolving.
How does the “what nursing means to me” reflective essay differ from a nursing school personal statement? Part of the answer is that a personal statement can include many different details from the past and does not have to take the form of a standard essay – introduction, thesis statement, supporting arguments. You can write a personal statement without including anything about your professional achievements or anything directly related to nursing. But a “what nursing means to me” reflective essay is the complete opposite. It is aimed at exploring how being a nurse or nursing student has directly impacted your worldview.
Depending on where and why you are asked to write a “what nursing means to me” reflective essay, there may be certain content and style guidelines you must follow, based on that program’s requirements. Some reflective essays use prompts or questions about nursing to give you a starting point for what to write. In these cases, how you write is up to you, but there are certain elements that are key to every outstanding reflective essay that you should incorporate into your writing. Here, we list elements you can include in the body of your essay to create a template of what your essay should look like.
The Inciting Incident
In university, reflective essays tend to revolve around coursework and materials to show how you’ve interacted with them. But according to many nursing essay writing services , a “what nursing means to me” reflective essay can structure itself around a catalyzing event. A traumatic injury. A death in the family. Moving to a new country.
If you haven’t been given instructions on what to write about in your “what nursing means to me” reflective essay, then you can choose a specific, life-altering event in your past that had and continues to have an impact on you. Many reflective essays take this path, but you do not have to, especially if you are asked to write about something else.
If you do write about a specific incident, be as specific as possible. Who did it involve? Where did it take place? What was the lead-up like? Of course, if you have word limits, then you can cut out unnecessary parts in your second draft, but for your first draft, you should be as detailed as possible and then make cuts later.
Personal Reflections
The most obvious point of a reflective essay is to reflect. Nursing school admissions officers want to know how you interpreted, compartmentalized, and dealt with an extreme situation related to being a health care worker, since everyone knows that being a health care worker exposes you to things most people never see. You can talk openly about what you’ve experienced in the nursing profession or during your schooling and, finally, express emotions you were not able to at the time. However, those emotions must also connect with an action you took later on to show that they led to some deep-seated change in your personality or outlook.
Specific Details
The topic of your essay should focus on a specific time, place, or incident and not stray into generalities. You want to place your readers in a setting they can understand, so that they can see the story as it unfolds. Remember: show, don’t tell. This is your personal story, so enrich it with specific details, like the time of year, the weather outside, some important event that happened at the same time, anything that makes the universal personal.
Your Actions
After you set the scene with vivid descriptions and a concrete location, you can start to bring yourself into the narrative. Take a step-by-step approach to write about how the specific event unfolded – before/during/after – and make sure to detail as much as you can about each step.
You can write about what your expectations were beforehand and what happened afterward. What was the aftermath like? What did you feel, physically, emotionally? What did you do? Were you in any danger? You can talk both about your physical and emotional reactions but remember that a reflective essay should walk a fine line between being a subjective and objective retelling of a major event so do not go too far either way.
Academic Details/Achievements
The reflective essay sits apart from other documentation like a nursing school recommendation letter and is not a place for you to recite your professional or academic CV. All the information about your academic achievements (GPA, transcripts) has already been reviewed, so the audience is not interested in hearing about it again.
Your reflective essay is a space for you to encapsulate something about yourself in a calm, measured tone. Of course, you can mention things about school that impacted you personally or an event that took place during your education, but reciting your CV looks boastful and misses the point of the reflective essay.
Too Many Emotions
The “what nursing means to me” reflective essay can contain many powerful emotions, but the language should always remain coherent and precise. You do not want to dwell on positive or negative emotions but instead, use them to push along your overall narrative. You can explain about how something made you feel, but then transition to what actions those emotions made you take.
Introduction
The introduction of any essay should present the topic about which you are going to write and hook the reader with an intriguing line. Afterward, you can briefly mention where you are in your career (registered nurse, nursing school applicant, grad school candidate) for context, but most of your introduction should be about the subject matter at hand, like the event you want to talk about or the general themes you want to explore.
You can also present your thesis in the introduction or keep it for a later paragraph if you want to structure your essay that way. After the introduction, you can begin to narrow your focus down to the inciting incident and move closer to finalizing your argument. You can also begin inserting background elements of your narrative like when and where it happened.
Thesis Statement/First Paragraph
If you put your thesis in the introduction, you can begin positing your arguments supporting it in the first paragraph of the body section. But you can also put your thesis here and begin expounding on your arguments in the proceeding sections. If you are writing about a specific event, this is where you would set the stage for what happens next.
Build your narrative toward a climax by describing where you were, what you were doing, and what expectations you had. When you reveal what happened, use that as a springboard to talk about the aftermath – how the event affected you and what you began to understand about it – which is what will lead you to your second paragraph.
Second Paragraph
The second paragraph can contain the “reflective” part of your essay, where you unpack the event and examine how it impacted you. You want to mention how you changed and how you began to see things differently while also expounding on the potential growth that this moment presented for you as a person and as a health care professional.
Conclusion
The end of your essay should tie into the thesis and summarize the best argument supporting it. You can draw from the body section to make the connections between your thesis and the other sections and ultimately land on a conclusion. It should reveal something significant you learned or realized because of that event and how it continues to shape you.
“What Nursing Means to Me” Reflective Essay Samples
“what nursing means to me” reflective essay sample #1 .
Things came into focus when I started coughing up blood. Up until then I was sure things would get better and that the danger would pass. Even though a doctor had told me I needed to be hospitalized, I thought nothing was wrong and that I would be okay. I told him to give me some antibiotics and send me home. But from one minute to the next, in the space of a heartbeat, something gave way, and I would not be the same again.
I have been lucky most of my life. I had never been seriously ill or broken anything. I had family members succumb to illnesses, but I hadn’t so much as scraped an elbow. I never thought about my fragility and vulnerability until I was forced to reckon with them when I was hospitalized with pneumonia.
Going to the hospital in an ambulance for the first time was nerve-wracking. I lay in the gurney with an oxygen mask over my face as the paramedics looked at my vital signs. I was feverish, short of breath, and still coughing, so there was barely any time to think or reflect. Things had progressed so fast that I had left my house still in my pajamas.
One of the first things that happened when I got to the hospital was that the attending nurse took my hand and told me, “You’re in good hands now, darling.” It was such a small gesture. She must have done it for everyone who came in, terrified, scared, and lonely. She knew what they were feeling, which is why she said it to everyone.
I spent almost two weeks in the hospital recovering, but that nurse came to see me every day and spent time with me, even with her busy schedule. She made the time. It was after one of her visits that the thought of being like her one day came into my mind. I was in my first year of undergrad and still hadn’t thought about my future.
The experience of being seriously ill made me realize how much we depend on the strangers who have committed their lives to helping the infirm. It is a vital role that nurses play in helping the ill feel whole again, often more so than doctors, because nurses spend more time with patients than any other health care professional.
The nurse who greeted me at the hospital entrance also helped me bring things into focus. Seeing up close the damage an illness like pneumonia can cause was one wake-up call, but the nurse who comforted me when I felt like the world was ending also woke me up. She met my fear and anxiety with compassion and understanding. She created the mold that I now wish to fill with my career and advancement in the nursing profession.
It was bright and sunny the day my mother died. She died in the early morning, and when I looked out the window of her room – she died at home – I could see people waiting for the bus, going on their way to work, completely unaware that I had lost the most important person in my life.
I wanted to go outside, tears streaming down my face, and tell everyone my mother had died. I wanted to spread my grief. I wanted the world to stop and acknowledge my loss. Grief was something new, and I didn’t know how to manage it.
I went on anti-depressants and began to self-medicate as well because I couldn’t stand the pain. I was going down a self-destructive path, and no one in my immediate family knew what to do. It was only through circumstance that my path was corrected before it led farther into darkness.
One afternoon, months after my mother’s death, I was at her house, arranging and organizing her belongings, when the palliative care nurse who had treated my mother at home came by to collect the medical supplies that had been left behind. It was pure luck that I was at home when she came.
I answered the door and invited her in. I explained what I was doing, and she told me why she was there. We went to the kitchen because she said she had some papers for me to sign. I don’t know why exactly, but between putting the kettle on for some tea and signing the papers, I started to cry. It was something I had been doing regularly, breaking down in public or suddenly, without warning. But that day, I showed my vulnerability to the right person.
The nurse, named Sylvia, rose immediately and gave me a hug. She held me as I wept and then began to tell me that she still mourns her sister. Her sister had died from the same illness as my mother and, according to Sylvia, had suffered just as much. Sylvia told me that she had dealt with her sister’s passing by pushing herself through nursing school. She wanted to memorialize her sister’s memory through the work she does every day as a nurse. Instead of letting her sister’s death tear her apart, like my mother’s death was doing to me, Sylvia took motivation from it and committed to becoming a person who helps people transition from life to death.
Sylvia told me that the pain never goes away, but it does lessen and when you are helping save lives and taking care of the most vulnerable people, it lessens even more. That day, Sylvia showed me the heights nurses can reach through their empathy and knowledge, not just as nurses but as human beings. Sylvia’s strength was my scaffold during an unsteady time, in addition to being the light that inspired me to become a nurse myself.
The thoughts in a reflective essay are your own, so you should not shy away from saying as much as possible, like the fact that you were afraid or felt negative emotions like anger, hatred, or despair. This is what the reflective essay is all about. If your essay is about a specific event in your past, then you should write about it in detail. You can allude to it in your introduction and build up to it through your main paragraphs, or not mention it at all in your introduction and reveal it later.
Being any kind of health care worker is demanding and stressful, but it can also be rewarding and humbling. The “what nursing means to me” reflective essay is the perfect way to talk about a moving or troubling incident in the past if you hadn’t had the chance to explore it before. It is not always possible for nurses and other health care professionals to express the emotions that come with the job, and working with a nursing essay writing service can help you craft a truly impressive account of an event that marked you deeply.
This essay is meant to help you express what is most important to you about being a nurse and reveal how you stay motivated.
The purpose of the essay is to let nursing school admissions officers know how you understand and interpret the nursing profession and what aspects of it appeal to you the most.
You can structure your essay around a specific event and then describe what happened before, during, and after this event. You can also talk about why this event means so much to you or what about it reflects the best aspects of nursing.
You should think about the reasons you wanted to become a nurse and what about your character or personality will sustain you throughout your career.
The length of your essay depends on the requirements of your particular program or school, since they all have different requirements.
A personal statement is a general background story of why you want to pursue a degree or get into nursing school, but a reflective essay specifically asks you to pick a particular moment and think about how it affected your career. So, a reflective essay is the reverse of a personal statement: rather than relating how personal experiences have shaped your personality, you write about how being a nurse has changed you personally.
A letter of intent looks at how your education, work experience, and anything else related to the nursing profession has prepared you to become a nurse, and also asks what you plan to achieve. A reflective essay does not focus on academic achievements but asks you to interpret a moment in the past.
Not all nursing schools or graduate programs will ask for a reflective essay. Admission requirements are different for every school, so make sure you know what they are before you apply.
Want more free tips? Subscribe to our channels for more free and useful content!
Apple Podcasts
Like our blog? Write for us ! >>
Have a question ask our admissions experts below and we'll answer your questions, get started now.
Talk to one of our admissions experts
Our site uses cookies. By using our website, you agree with our cookie policy .
FREE Training Webclass:
How to make your nursing school application stand out, and avoid the top 5 mistakes that get most rejected.
Communication in Nursing Practice: Gibbs’ Reflective Cycle Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
Description, action plan, reflective conclusion.
Communication is a fundamental element in nursing practice. This element can possibly determine patients’ satisfaction and even the outcomes of their treatment (Lotfi et al., 2019). The situation described in the paper will exemplify the potential role of communication, which is why it will serve as a Gibbs Reflective Cycle nursing example. The cycle will help to assess the situation and extract lessons from it.
The model is a widely-recognized and crucial learning instrument, allowing individuals to extract lessons from life experiences. The pattern helps one to consider previous experiences, reevaluate them in the light of new knowledge, and implement the freshly obtained insight to improve future practice (Markkanen et al., 2020). The cycle is composed of six stages (description, feelings, evaluation, analysis, conclusion, and action plan), on which the reflection regarding the personal experience will be based (Markkanen et al., 2020). The paper’s principal objective is to outline a challenging situation from personal practice using Gibbs’ Reflective Cycle. The problematic situation is an encounter with a patient suffering from an infected diabetic foot ulcer and in need of amputation. Overall, the paper aims to critically analyze the situation and transform it into a learning opportunity useful in improving my future practice as a wound care specialist.
The situation concerns a 40-year-old patient with diabetes and an infected foot ulcer who was admitted to the hospital where I was working at the moment. The patient had a long history of diabetes, from which he suffered since he was ten years old. A multidisciplinary team examined the patient and established that he needed an amputation. As I approached the patient to get a consent form, I noticed that he looked upset. Given the described situation, it might be suggested that a communication dilemma here is of ethical character, in particular – it is the delivery of the bad news. By applying the model, the provided Gibbs Reflective Cycle example communication will demonstrate what actions were undertaken to resolve the mentioned dilemma.
The incident that will be analyzed is an outstanding Gibbs Reflective Cycle nursing example, which happened several years ago when I began working as a wound care nurse. A 40-year-old diabetic patient with an infected diabetic foot ulcer was admitted to the hospital. He had a long history of diabetes, suffering from the condition for three decades. A multidisciplinary team examined and communicated with the patient; it was established that he needed a below-knee amputation. The group stated their decision and left, and I had to retrieve the consent form. While retrieving the record, I perceived that the patient looked exceedingly sorrowful and depressed. Nevertheless, I did not know whether I needed to intervene in the situation and left.
Although I worked for many years in nursing before the incident, I became a certified wound care nurse relatively recently before it took place. At the moment, I saw the situation as irreparable, so I was not sure whether I should have tried to console the patient. I felt anxious and, to an extent, powerless when faced with the man’s grief. I thought that words or an empathic response would not be able to mitigate his sadness. Additionally, I was also somewhat startled that the multidisciplinary team did not handle the conversation more delicately and left rather abruptly. Overall, I did not feel confident enough to handle the situation and was unsure whether my intervention would be appropriate.
I frequently returned to the incident, trying to understand what should have been done instead. Retrospectively, I believe that it helped me to reevaluate the role of therapeutic communication in my profession. Prior to the incident, I did not perceive preoccupation with patients’ emotional well-being as my duty as a nurse. I believed that administering medications and treatment, performing tests, recording medical history, educating patients, et cetera, was all that was required of me. Nevertheless, I did not fulfill another vital function in the described situation. To understand that a holistic approach to care presupposes therapeutic communication, I had to experience the case (2). As a nurse, showing empathy and consoling patients is a critical function that is sometimes overlooked. Furthermore, the incident demonstrates a lack of cooperation between the nursing staff and the team since communication was needed to ensure that the emotional impact of amputation on the patient was alleviated.
Some medical professionals find the process of delivering bad news challenging and feel psychologically unprepared (Van Keer et al., 2019). A lack of skills in this aspect can negatively affect patients: they might undergo extra stress, have lower psychological adjustment, and have worse health outcomes (Biazar et al., 2019; Matthews et al., 2019). Furthermore, the way the news is handled can impact patients’ understanding of the situation and adherence to treatment (Galehdar et al., 2020). Given the adverse effects, multiple protocols and approaches to communicating bad news and dealing with its consequences were developed. This situation is analyzed in detail in a ‘Gibbs Reflective Cycle example essay pdf’ that focuses on these communication challenges in healthcare.
In the patient- and family-centered approach, the process occurs based on the patient’s needs as well as their cultural and religious beliefs (Hagqvist et al., 2020). Upon communicating the information, a medical professional is supposed to assess their understanding and show empathy (Hagqvist et al., 2020). In an emotion-centered approach, a medical professional is supposed to embrace the sadness of the situation and build the patient-medical professional interaction on empathy and sympathy (Hagqvist et al., 2020). Yet, the patient- and family-centered approach seems more effective since excessive empathy can be counter-productive and impede information exchange.
Managing patients’ reactions is the final and particularly vital step in communicating bad news. Nurses are commonly involved in handling emotional responses, which entails several responsibilities:
- Additional emotional support should be given to those who cannot accept the information (Galehdar et al., 2020).
- Nurses can find more related information and share it with patients (Rathnayake et al., 2021).
- Nurses are supposed to improve the situation if bad news has been delivered poorly (Dehghani et al., 2020).
In the case of amputation, heightened emotional attention should be given to the patient, as limb loss is a life-altering procedure. Such patients commonly undergo the five stages of grief (denial, anger, bargaining, depression, and acceptance) and are prone to developing anxiety, depression, and body image issues (Madsen et al., 2023). Hence, upon delivering the news regarding amputation, it is vital to provide a patient with community resources for dealing with emotional and psychological implications.
Currently, I understand more in-depth that delivering and handling the consequences of bad news is an inescapable reality of the nursing profession. The incident allowed me to notice the aspects of my professional development that necessitate more attention and improvement. Hence, I strive to be more empathetic in my clinical practice and not undervalue the role of patient-nurse communication. I attempt to provide psychological and emotional support to patients and console them to the best of my ability and knowledge, especially if a patient has just received traumatic news. Due to the incident, I comprehended better that a patient’s emotional well-being can be dependent on my actions. I also stopped presuming that other medical professionals provide the necessary emotional support. Moreover, I understand that I am not powerless when faced with a patient’s sorrow.
Consequently, I will not neglect the importance of patient-nurse communication for patients’ health outcomes and mental well-being. I will offer hope where it is appropriate and encourage and validate patients’ emotions to help them deal with traumatic information (Font-Jimenez et al., 2019). In the future, I will use verbal and non-verbal communication clues to show that I care and, generally, be more empathetic (Font-Jimenez et al., 2019). I will not prevent my insecurities from fulfilling my nursing duties, nor will I allow the feeling of hopelessness to affect my clinical practice. Furthermore, I will rely on evidence-based approaches to handle bad news effectively and facilitate its delivery to patients.
Additionally, I will be more mindful in my nursing practice. Gibb’s reflective cycle will assist me in attaining this objective. I will continue to apply it to the situations occurring at work in order to think systematically as well as analyze and evaluate them. Furthermore, Gibb’s reflective cycle will enhance my ability to learn from my experience. The model will help me to refine my communication skills and make patient-nurse interactions more intuitive and productive (Markkanen et al., 2020).
The situation allowed me to understand the actual value of therapeutic communication in nursing. Now, I understand the need to exercise it in my clinical practice, which is a realization that I further explored in a ‘Gibbs Reflective Cycle example essay pdf.’ Learning to provide emotional support and manage the consequences of bad news is an essential quality for nurses, influencing health outcomes and satisfaction from a visit. Additionally, I become more conscious of my own emotions and the way they can prevent me from acting in a patient’s best interests. Overall, the proper tactics of delivering bad news and assisting patients in handling them became a higher priority in my clinical practice.
To conclude, this reflection featured an episode from my practice in which I analyzed a communication situation using Gibbs’ Reflective Cycle. It showed that I need to concentrate on my abilities to resolve the communication dilemma of the delivery of bad news. The above discussion also demonstrated how the implementation of an appropriate and significant evidence-based model – Gibbs’ Reflective Cycle – may result in better patient outcomes.
Biazar, G., Delpasand, K., Farzi, F., Sedighinejad, A., Mirmansouri, A., & Atrkarroushan, Z. (2019). Breaking bad news: A valid concern among clinicians . Iranian Journal of Psychiatry, 14 (3), 198–202. Web.
Dehghani, F., Barkhordari-Sharifabad, M., Sedaghati-kasbakhi, M., & Fallahzadeh, H. (2020). Effect of palliative care training on perceived self-efficacy of the nurses . BMC Palliative Care, 19 , 63. Web.
Font-Jimenez, I., Ortega-Sanz, L., Acebedo-Uridales, M. S., Aguaron-Garcia, M. J., & de Molina-Fernández, I. (2019). Nurses’ emotions on care relationship: A qualitative study . Journal of Nursing Management, 28 (8), 2247-2256. Web.
Galehdar, N., Kamran, A., Toulabi, T., & Heydari, H. (2020). Exploring nurses’ experiences of psychological distress during care of patients with COVID-19: A qualitative study . BMC Psychiatry, 20 , 489. Web.
Hagqvist, P., Oikarainen, A., Tuomikoski, A.-M., Juntunen, J., & Mikkonen, K. (2020). Clinical mentors’ experiences of their intercultural communication competence in mentoring culturally and linguistically diverse nursing students: A qualitative study . Nurse Education Today, 87 , 104348. Web.
Lotfi, M., Zamanzadeh, V., Valizadeh, L., & Khajehgoodari, M. (2019). Assessment of nurse–patient communication and patient satisfaction from nursing care . Nursing Open, 6 (3), 1189-1196. Web.
Madsen, R., Larsen, P., Carlsen, A. M. F., & Marcussen, J. (2023). Nursing care and nurses’ understandings of grief and bereavement among patients and families during cancer illness and death – A scoping review . European Journal of Oncology Nursing, 62 , 102260. Web.
Markkanen, P., Välimäki, M., Anttila, M., & Kuuskorpi, M. (2020). A reflective cycle: Understanding challenging situations in a school setting . Educational Research, 62 (1), 46-62. Web.
Matthews, T., Baken, D., Ross, K., Ogilvie, E., & Kent, L. (2019). The experiences of patients and their family members when receiving bad news about cancer: A qualitative meta-synthesis . Psycho-Oncology, 28 (12), 2286-2294. Web.
Rathnayake, S., Dasanayake, D., Maithreepala, S. D., Ekanayake, R., & Basnayake, P. L. (2021). Nurses’ perspectives of taking care of patients with Coronavirus disease 2019: A phenomenological study. PLoS ONE, 16 (9), e0257064
Van Keer, R. L., Deschepper, R., Huyghens, L., & Bilsen, J. (2019). Challenges in delivering bad news in a multi-ethnic intensive care unit: An ethnographic study . Patient Education and Counseling, 102 (12), 2199-2207. Web.
- Root Cause Analysis of Decubitus Ulcers
- Nurse Practitioner Cost Effectiveness
- Gibbs’s Reflective Cycle and Borton’s Model of Reflection
- "The Epic of Gilgamesh" by Ryan Gibbs
- The Topic of Diagnostic Measures
- Modern Nurse’s Role: Leadership
- Nurse Leader and Abbott Northwestern Hospital
- Mindfulness Meditation Program and Nursing Outcomes
- Catheter-Associated Urinary Tract Infection: Nurse-Sensitive Indicators
- Healthcare Quality and Safety in Practice
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, June 17). Communication in Nursing Practice: Gibbs' Reflective Cycle. https://ivypanda.com/essays/gibbs-reflective-cycle-essay-essay-examples/
"Communication in Nursing Practice: Gibbs' Reflective Cycle." IvyPanda , 17 June 2022, ivypanda.com/essays/gibbs-reflective-cycle-essay-essay-examples/.
IvyPanda . (2022) 'Communication in Nursing Practice: Gibbs' Reflective Cycle'. 17 June.
IvyPanda . 2022. "Communication in Nursing Practice: Gibbs' Reflective Cycle." June 17, 2022. https://ivypanda.com/essays/gibbs-reflective-cycle-essay-essay-examples/.
1. IvyPanda . "Communication in Nursing Practice: Gibbs' Reflective Cycle." June 17, 2022. https://ivypanda.com/essays/gibbs-reflective-cycle-essay-essay-examples/.
Bibliography
IvyPanda . "Communication in Nursing Practice: Gibbs' Reflective Cycle." June 17, 2022. https://ivypanda.com/essays/gibbs-reflective-cycle-essay-essay-examples/.
📕 Studying HQ
How to write a nursing reflection paper [+nursing reflective essays], bob cardens.
- August 23, 2022
- Essay Topics and Ideas , Nursing
Reflective writing is a skill that every nurse should learn. As health professionals, we are constantly confronted with ethical decisions that we must make. In order to be effective in our roles, we need to be able to think critically and reflect on our experiences. This reflection can be done through journaling , but it can also be done through writing reflective papers. Here’s a guide on how to write a nursing reflection paper and nursing reflective essays that are both effective and meaningful.
What You'll Learn
What is a reflection paper?
A reflection paper is a type of writing that helps you to look back on your experiences in school, work, or life and to think about the ways in which you have grown or changed as a result. A reflection paper can be a simple document that you write yourself, or it can be a more formal piece that you submit as part of a coursework assignment . Reflection papers can be helpful in several ways.
They can help you to reflect on your experiences and to take stock of what you have learned . They can also help you to change or improve your behaviour in future situations.
There are many different ways to write a reflection paper , and the format will depend on the context in which it is being written. However, there are some general principles that all reflection papers should follow.
- Reflect on what you have learned in the past year or so.
- Take stock of your accomplishments and failures during this time period.
- Think about how your experiences have changed you as a person.
- Identify any skills or knowledge that you have acquired during this time period that you would like to share with others.
As you continue, thestudycorp.com has the top and most qualified writers to help with any of your assignments. All you need to do is place an order with us.
How to write a reflection paper in nursing
Reflection papers are important for nurses to complete as part of their professional development . They can provide nurses with a unique opportunity to reflect on their own experiences, identify strengths and weaknesses, and develop plans for future development. Here’s a guide on how to write a nursing reflection paper and nursing reflective essays that are both effective and meaningful.
1. Prioritize your reflection When writing a reflection paper , it is important to prioritize your thoughts and feelings. Start by listing the main points that you want to address in your essay. Next, focus on detailing these points in a clear and concise way. Finally, be sure to provide a conclusion that ties all of your ideas together.
2. Assess your own skills Before you can identify any areas where you need improvement, it is important to assess your own skills and strengths. Include questions like the following in your reflection paper: What have I done well in my career as a nursing student ? What do I enjoy doing most in my work as a nurse? How have my performances as a nurse impacted patient care ? How have I developed as a professional over the past year ? This information will help you identify areas where you are strongest and should continue developing those skills. It will also allow you to identify any areas where you may need additional training or practice.
3. Reflect on your experiences When you are writing a reflection paper , it is important to think about the emotions that you experienced during the events that you discuss. This can be difficult, but it is important to remember that emotions are a part of every human experience . Try to be as candid as possible while still maintaining a degree of objectivity.
4. Analyze your experiences When you are writing a reflection paper , it is important to analyze your experiences in order to understand them better. Ask yourself questions like the following: What were some of the challenges that I faced during this time period? How did I respond to these challenges? What did this experience teach me about myself? What did this experience teach me about my profession? How can I use what I have learned from this experience in the future? By analyzing your experiences, you will be able to better understand both the good and bad moments from your past year and career as a nurse.
Reflection papers can be a helpful tool in nurse development . By following these tips, you will be able to write an effective and meaningful reflection paper .
Reflection Paper Example
Nursing Leadership Style Reflective Essay Assignment
Personal and Professional Accountability in Nursing
Opportunity Analysis and Plan for Professional Development
What should be included in a reflection paper?
Reflection papers should include an overview of the patient’s care and a personal reflection on what was learned during the course of that care. Additionally, reflection papers should address specific issues or questions that arose during the patient’s stay in the hospital setting. Finally, reflection papers should offer any suggestions or advice that the nurse may have for improving patient care in the future.
Tips for writing a reflection paper in nursing
Reflection papers are a great way to document your learning in nursing . They can be used as an important tool to evaluate your own progress and growth as a nurse or to share your thoughts and experiences with others.
Here are some tips for writing a reflection paper in nursing:
- Think about what you want to communicate in your reflection paper. There are many different types of reflection papers, so think about what would be most helpful for you as a nurse. Some common focuses include assessment of skills, clinical experiences , professional development, and personal growth.
- Start by writing down your thoughts and feelings about the experience or class you’re writing about. This will help you to organize your thoughts later on in the paper.
- Take time to reflect on the content of the class or experience you’re reflecting on. What did you learn? What did you enjoy? What challenges did you face ? How did you overcome them? These are all important questions to ask yourself when writing a reflection paper.
- Choose a specific topic or theme to focus on in your reflection paper , and then research related materials on that topic. This will help you to develop a well-rounded perspective on the topic and to communicate that perspective effectively in your paper .
- Organize your thoughts into logical sections, and then write prose that is clear, concise, and easy to read. Use concrete examples to illustrate your points, and stay away from theological or philosophical rambling.
Writing a reflective paper can be difficult, but it is an important part of nursing. A reflective paper should help nurses learn from their experiences and grow as professionals . By reflecting on the care that they provide, nurses can identify areas for improvement and work to make changes in order to provide the best possible care for their patients. Here’s a guide on how to write a nursing reflection paper and nursing reflective essays that are both effective and meaningful.
How to write a reflection essay in Nursing
Nursing is an incredibly challenging and rewarding career choice. In this article, we will explore the basics of writing a reflective essay , which is a type of writing that helps you reflect on your experiences as a nurse and consider what it has taught you about yourself. We will provide tips on how to structure the essay , outline key points, and give you some ideas for reflective quotes to use.
What is a reflection essay?
When you write a reflection essay , you are writing about your own life experiences and how they have affected you. A reflection essay can be about anything that has affected your life, from a personal experience to something you have read or seen. The purpose of a reflection essay is to allow you to explore your thoughts and feelings about what has happened in your life and to learn from them.
How do I write a reflective essay?
There is no one way to write a reflection essay . What matters most is that you take the time to reflect on your life experiences and share what you have learned from them. You can start by brainstorming some ideas for topics that might interest you, then begin writing down your thoughts and feelings. Be honest with yourself- if something feels uncomfortable or difficult, try not to censor yourself. Let the words flow out of you without restraint.
What are the steps of writing a reflective essay?
There are a few steps to writing a reflective essay in nursing . First, you should assess the importance of reflection for your career. Second, identify what you learned from your experiences in nursing. Third, document your reflections in a cohesive and meaningful way. Fourth, share your reflections with others to gain feedback and improve your skills as a nurse .
As you continue, thestudycorp.com has the top and most qualified writers to help with any of your assignments. All you need to do is place an order with us. Here’s a guide on how to write a nursing reflection paper and nursing reflective essays that are both effective and meaningful.
Guidelines for writing a reflection essay
Reflection essays are common assignments for nursing students. They can offer a chance to reflect on their own learning and growth as nurses, and to share that experience with others. Here are some tips for writing a reflective essay:
- Choose a topic that interests you.
- Write about a specific experience or event from your nursing career.
- Consider how the experience has affected you personally.
- Share what you learned from the experience, and why it matters to you as a nurse.
- Reflect on how your experiences have shaped who you are as a nurse today.
- Share any advice you have for other nurses who are struggling to find their path in the profession.
- Thank your readers for taking the time to read your essay.
Tips to help you write a reflective essay
Writing a reflective essay can be a difficult task, but with the right tips, it can be a rewarding experience. Here are four tips to help you write a reflective essay effectively:
- First, make sure that your essay is focused. Make sure that each paragraph addresses a specific point of your reflection. If your essay is scattered or unfocused, it will be more difficult to write effectively.
- Next, be honest with yourself. Don’t try to sugarcoat your thoughts or hide the truth behind your reflection. Be vulnerable and open up about what you’ve learned from the experience. This will help readers understand and connect with your essay.
- Finally, use descriptive language when describing your experience. Use concrete images and words to help readers imagine what you’re talking about. This will help them understand the emotions that you were feeling during the experience.
These tips should help you write a reflective essay that is both effective and enjoyable to read. Here’s a guide on how to write a nursing reflection paper and nursing reflective essays that are both effective and meaningful.
What to include in your reflection essay
In order to write a reflection essay on nursing , you will need to think about what inspired you when you initially became interested in the field. Why did you choose nursing as your chosen profession? Reflect on the reasons why you are passionate about this career and share them with your readers. Additionally, make sure to describe the important aspects of your nursing experience and how it has shaped who you are as a person. Finally, be sure to offer advice for aspiring nurses out there.
How to write a reflection essay in Nursing?
Reflection is a necessary part of nursing. It allows nurses to think critically about their practice and how it can be improved. Reflecting on experience also allows nurses to develop self-awareness. When writing a reflection essay , it is important to follow the three steps analysis, reflection and evaluation.
In order to begin the reflective process, nurses should first assess their experience. This can be done by thinking about the specific aspects of care that were observed during the writing process . For example, did you feel empowered as a nurse during your reflections? Were there any areas in which you felt unsure or uncomfortable? Once the experience has been assessed, it is important to begin reflecting on it.
In order to understand why things happened and what lessons could be learned from the experience, nurses need to reflect on their thoughts and feelings at the time of the event. This includes everything from recalling what was going through your mind as you performed the task to analyzing why certain decisions were made. It is important, to be honest with yourself, and allow your thoughts and feelings to surface without censorship.
After reflecting on the experience, it is important to evaluate what was learned. This can take many different forms, such as writing a list of positive and negative outcomes that came from the experience. It is also important to think about ways in which the experience could have been improved. By evaluating the experience, nurses can learn from their mistakes while still making progress.
Nurses should always keep in mind the three steps of reflection when writing a reflective essay. By following these steps, nurses can develop a deeper understanding of their practice and learn from their experiences.
Reflection essays are a powerful tool for nursing students to use when reflecting on their experiences in the profession. They can provide insight into how nursing has contributed to your personal growth and development, help you identify areas where you need to continue developing as a nurse , and provide you with opportunities to share your clinical skills and knowledge with others.
When done well, reflection essays can provide nurses with a unique perspective on their career path and help them see both the good and bad moments of their experience in the profession .
Start by filling this short order form order.studyinghq.com
And then follow the progressive flow.
Having an issue, chat with us here
Cathy, CS.
New Concept ? Let a subject expert write your paper for You
Have a subject expert write for you now, have a subject expert finish your paper for you, edit my paper for me, have an expert write your dissertation's chapter, popular topics.
Business Analysis Examples Essay Topics and Ideas How to Guides Nursing
- Nursing Solutions
- Study Guides
- Cookie Policy
- Free College Essay Examples
- Privacy Policy
- Research Paper Writing Service
- Research Proposal Writing Services
- Writing Service
- Discounts / Offers
Study Hub:
- Studying Blog
- Topic Ideas
- How to Guides
- Business Studying
- Nursing Studying
- Literature and English Studying
Writing Tools
- Citation Generator
- Topic Generator
- Paraphrasing Tool
- Conclusion Maker
- Research Title Generator
- Thesis Statement Generator
- Summarizing Tool
- Terms and Conditions
- Confidentiality Policy
- Cookies Policy
- Refund and Revision Policy
Our samples and other types of content are meant for research and reference purposes only. We are strongly against plagiarism and academic dishonesty.
Contact Us:
📧 [email protected]
📞 +15512677917
2012-2024 © studyinghq.com. All rights reserved

ANA Nursing Resources Hub
Search Resources Hub

Nurturing Leadership: 4 Key Strategies for Reflective Practice in Nursing
5 min read • February, 21 2024
Nursing leaders play a pivotal role in guiding change to enhance patient care and improve professional practice environments. Reflective practice stands out as a cornerstone for effective leadership, allowing nursing professionals to evaluate their experiences critically and foster continuous improvement. This article delves into four key reflective practices that can empower nursing leaders to drive meaningful change.
The Power of Reflection in Nursing Change
Reflective practice is more than a method; it's a mindset that enables nursing leaders to learn from their actions, make informed decisions, and engage their teams in the journey towards excellence. By incorporating reflective practices into their leadership approach , nurses can better navigate the complexities of healthcare, adapt to challenges, and implement strategies that align with their goals and values.
Understanding Your Change Goals
Q: What did you say you were going to do? A: Begin by revisiting your initial objectives. Clear articulation of your goals lays the foundation for accountability and sets the stage for impactful change. Reflect on the scope of the changes you envisioned and the outcomes you aimed to achieve. This honest appraisal is your first step towards meaningful progress.
Assessing Your Actions
Q: What did you actually do? A: Reality often diverges from our plans. Assessing your actions with candor enables you to identify discrepancies between your intentions and your actual practices. Acknowledge both your achievements and the areas where you fell short. This recognition is crucial for realistic self-assessment and sets the groundwork for authentic growth.

Learning from Experience
Q: What did you learn? A: Every step in the change process offers valuable lessons. Reflect on the insights gained from your experiences and how they can inform future strategies. These lessons are the silver lining, providing clarity and direction for your next moves.
Planning Your Next Steps
Q: What do you need to do next? A: Armed with new knowledge, plan your forward strategy. Consider who needs to be involved, the resources required, and the timeline for implementation. This step is about translating insights into actionable plans that drive further change.
Incorporating Reflective Practice into Your Routine
Integrating reflective practice into your leadership routine doesn't have to be daunting. Start small with regular reflection sessions, encourage team discussions that foster collective learning, and set aside time for personal and professional development. Embracing reflection as a habit can transform your leadership approach and significantly impact your team's performance and well-being.
Reflective practice is an invaluable tool for nursing leaders seeking to navigate the complexities of healthcare and drive positive change. By focusing on these four essential aspects of reflection, you can enhance your leadership effectiveness, improve patient care , and foster a culture of continuous learning and improvement . Start today by taking a moment to reflect on your practice and empower yourself and your team for the challenges and opportunities ahead.
Images sourced from Getty Images
You May Also Like
Specialty Certifications
Presented by ANCC
Nurse Executive Certification (NE-BC®)
Price From: $395.00
ANA Member Price: $295.00
Nurse Executive, Advanced Certification (NEA-BC®)

Want to learn more?
Discover how ANA Consultation Services can help you maintain and improve nursing excellence by signing up for a FREE organizational assessment.
Related Resources

Item(s) added to cart
Academic Support for Nursing Students
No notifications.
Disclaimer: This reflective essay has been written by a student and not our expert nursing writers. View professional sample reflective essays here.
View full disclaimer
Any opinions, findings, conclusions, or recommendations expressed in this reflective essay are those of the author and do not necessarily reflect the views of NursingAnswers.net. This reflective essay should not be treated as an authoritative source of information when forming medical opinions as information may be inaccurate or out-of-date.
Reflection on Clinical Placement
Info: 2965 words (12 pages) Reflective Nursing Essay Published: 20th Oct 2021
Reference this
Reflecting on clinical placement
Clinical placement reflection 1.
- Communication with others within the clinical setting – this may include staff members, patients and relatives. From working within in a team I have learnt that communication in health and social care underpins everything that professionals do and can determine the quality of service that patients receive. Poor communication between members of inter-professional teams affects the quality of care patients receive and can result in any number of bad experiences for the patients. I’ve noticed that some of the factors that contribute to poor communication include the use of professional jargon, abbreviations and poor handwriting. Thus I made it a personal objective try and avoid these behaviours and if I did for example, use jargon I made sure its universal so that there is no miscommunication.
- Your ability to work within a team. During this time on placement I have been able to witness and participate in multidisciplinary team meetings and have also had the opportunity to work with different healthcare professionals such as physiotherapist and also have been involved in ward rounds with doctors and nurses. The ward I was placed had a great team. I always felt a part of the team, and that I was making a valuable contribution thanks to both my eagerness to take on anything they threw at me, and, their inclusiveness and supportiveness. I learnt that holding back and waiting for people to notice you is not a good strategy. Let everyone know that you’re interested every day, and opportunities will come your way.
- Your role and relationship with others within the clinical practice My role and relationship with others within the clinical setting was very important to me. It was important for me to build the team member relationship with others as it helps to establish a mutual understanding and trust with other team members. When reflecting upon my own strengths and weaknesses, in relation to my role and relationship with others, I feel that whilst I am beginning to acquire an in depth understanding of the role of the nurse in the care of the patient, I feel that I lack substantial knowledge of the roles of the other healthcare professionals that I work alongside in the care of these same patients. I have listed the priority of this as being medium to high as I feel that it is not possible to become an effective collaborative healthcare member without having an understanding of the roles of others with whom I am are working with. If there is a lack of understanding of the roles of others, I feel that it would be difficult to see why they are involved in the care of my patient, or even when it is appropriate to involve other healthcare professionals, inevitably leading to the quality of the care for the patient to suffer. Thus I made it in my best interest to interact more with others during the placement so that I could work alongside them confidently and work towards the same goals.
- Your problem solving skills. Where possible give examples of how you have used these. Being in acute care setting, the kind of problem-solving that I did tend to be solving were immediate problems rather long-term problems. For example, patients who can’t get up who needs to have a sponge and clean linens on their bed. However each patient is different and often I couldn’t just perform the task without thought. The process of problem solving has guided me in finding solutions to a problem. I have learnt that this process is effective and helps me make the right decisions. I have also learnt to reflect on my decision so that I can see if the decision was the best or not for the situation. To assist me in problem solving more complex situations, I asked my mentor to provide some problem solving stimulations to further develop my problem-solving skills which was very beneficial for my learning process.
- Your time management skills I learnt the importance of time management a lot during this placement. On one of the days, I did not wash my patient before 8:00 am in order to prepare her for her family visit later on that day. My patient’s 10:00 am Parkinson’s’ medication administration was delayed by 45 minutes because she was being washed at that time. This event occurred because I did not prioritize the tasks I had to complete during the day properly, and therefore I learned the importance of time management. If I had obtained nursing knowledge and familiarize myself with the daily routine care of the patient I could have handle my time more efficiently. After this event, I learned effective time management strategies to handle my time on clinical and I will definitely utilize the time managing strategies step by step from knowing , planning , prioritizing tasks, gaining support from other nurses, and being flexible with my tasks.
- Your ability to understand your competence and scope of practice It was important for me to have an understanding my own competence and scope of practice as it guides me to good clinical practice and the delivery of my care to my patients. As a student enrolled nurse, there were strict practice standards and regulations, especially when medication is involved and supervision from our mentor was required at all times. Reflecting back, there were situations where although the practice was within my scope of practice, the policy and procedures of the facility did not allow it thus it is important to understand and familiarize yourself with the policy and procedures of the hospital as well as working within your scope.
- How you adapt and use new technology and reflect on historical changes in nursing As I was quite young and I didn’t have any problems adapting and using new technology. It was very exciting seeing new technology as I believe it will greatly improve nursing practice and make it more efficient. Some of the new technologies I had the opportunities to use include new vital signs machine, lifting machines and computer programs that included online hospital policy and procedures and mims. All documentation was still hand-written and stored in folders. Historically, nursing documentation has been a hand- written. However, today facilities are now introducing new technology aimed to improve efficiency and effectiveness.
- Your competency in relation to your nursing skills Competency in relation to my nursing skills had a lot to do with my confidence. For me, confidence is an important aspect of the practice of nursing and plays a major role in development. After an incident, I recognised that despite my lack of confidence the skill set that required to be completed for a particular patient was within my scope of practice, within my ability and within my knowledge base. On reflection it is clear that my lack of confidence in my ability, when put ‘on the spot’ contributed to my feelings of inadequacy which affected my competency in relation to my nursing skills. Through reflection and use of a mentor I was able to clarify that my weakness was not my nursing skills or knowledge level but my inability to effectively perform when under close scrutiny, which in turn led to a lack of confidence in my skill level and clinical knowledge. To develop my confidence and communication skill I became proactive and requested the assistance of a senior clinical educator to critically assess my skills and knowledge base. By doing this I reinforced my knowledge and skills but more importantly developed self-confidence. My efforts in seeking assistance and assessment of my skills could be seen as a step in ‘becoming more independent’. By gaining input and validation from my clinical educator, I ensured that I was able to successfully utilize my nursing skills competently and independently.
- An overall reflection of the day and how you can use your reflection to improve your practice Upon starting this placement I didn’t really know what to anticipate as it was my first time in a hospital setting, so when I was asked about my expectations all I could say was “That I wanted to learn all I can…” Thinking back on that not so confident response, now at the end of this placement, gives me good insight into how valuable this placement has been to my professional development. This placement gave me the opportunity to begin to see myself as a professional, as I gradually learnt how to think critically about the patient’s health and managing their care and needs. This led to my becoming increasingly confident in the role I was determined to fill. Although I feel that I have a long way to go in this endeavour and will always continue learning, I know that I have learnt a lot during my time at this placement. To further improve my practice, I will use reflection as it enables me to solve problems or learn from actions through thinking consciously about an activity which would then enable me learn and develop.
Clinical Placement Reflection 2
- Communication with others within the clinical setting – this may include staff members, patients and relatives. During my placement I was in a stroke unit. Being on this unit, I worked with many clients who suffered from post-stoke symptoms and many had difficulty with speech. The experience helped me learn the importance of both verbal and non-verbal communication. As an aspiring nurse, I have to continuously sharpen my communication skills because I will be interacting with more diverse range of patients in the future. I have to be able to establish rapport with each new patient and I can do this by communicating with them. I must maximise my communication with my patients because I can do a lot of things by communicating such as motivate, empower, educate and understand my patients.
- Your ability to work within a team. Working in a team provides me with a great satisfaction. From this teamwork experience, I strongly feel the importance of teamwork in providing consistent care for patients. It taught me to interact with others and as an individual in order to work towards the same goal. I had witnessed examples of both good and poor inter-professional collaboration and communication whilst in practice and have reflected on those incidents. Also, being self-aware I found was not only essential to nursing as a basis for reflection but it also contributed to how I communicate with others and assisted me in developing inter-personal skills with our colleagues so that I could work better in a team.
- Your role and relationship with others within the clinical practice In order to work efficiently together, it was important to understand my role and the roles of others within the clinic setting. My professional relationships with others was good except with occupational therapist (OT) mainly because I never had a fully understand of their role. I felt more clarity was needed surrounding the role of the OT so I made the efforts to research and speak to some of the OT to find out about their roles. As a result I was able to witness some of their work and worked closely with them. Being professional relationships and understand the roles of others has also lead me to understanding about the unique qualities and skills that different professionals bring to the team. In learning about other team members roles, I was also able to enhance my own professional identity which made me comfortable in the clinical setting.
- Your problem solving skills. Where possible give examples of how you have used these. I was quite a confident problem-solving however there are times where I’m faced with a situation where I can identify the problem but I’m not able to come up with a solution due to lack of experience. For example performing venepuncture; the patient had very poor vein and my first attempt failed and was very demotivated. We were never taught in school on how to deal with bad veins except to have two attempts and have someone else do it if you fail. Determined to do the venepuncture successful, I suggested to my mentor, if I was able to pull the vein downwards to minimise the movement of the vein. With the approval of my mentor, I went in and was successful on my second attempt.
- Your time management skills Prioritizing is a necessary tool for effective time management thus I made sure I prioritize tasks from high to low priority order. However during this placement, a staff had questioned my time management skill because I choose to assist patient who was lying in a soiled bed over researching my medications that was due in half an hour. This experience made me question how I was prioritising my time management skills. I decided to reflect on experience, and I believe that the staff was wrong to question my time management skills. I had thought about which was the greater priority in this situation and I still believe that the patient was. If the same situation arose again I feel that I would not do anything different other than to speak up and justify my decisions. I recognize and knowledge Time management as an important skill in nursing and hope that my skills will further improve with practice.
- Your ability to understand your competence and scope of practice My ability to understand my competence and scope of practice is demonstrated through my clinical practice to safely provide care that is in accordance to the competency standards and actively informing others of my scope of practice. I always clarified what the specific activity or task details were from my mentor and obtained any additional information that I may need, such as facility policies or procedures. I also I made sure I expressed the concern to my mentor or educator. An example of this was my knowledge of medication. Although it was in my scope to administer medication, it was important that I do it competently with full understanding of the drug use and why the patient is receiving it. While it was in my scope, I lacked knowledge of the drug therefore I felt incompetent to administer it safely. For this reason, I made it my goal to research the medications prior to administration.
- How you adapt and use new technology and reflect on historical changes in nursing During my placement, I adapted well to the new technology. The ward I was on had new software integrated in their computers which allowed me to access patient results and continue my learning through web-based training programs and access to online medical dictionaries and mims. My experience using these was very positive and very efficient. Historically, nursing documentation has been a hand- written. This was still the case in the ward that I was working in. This form of documenting has provided good communication between health care professionals and plan of patient care for the patient for a long period however the increase of medical errors and miscommunication has driven facilities to introduce new technology aimed to improve this and the efficiency and effectiveness of clinical practice.
- Your competency in relation to your nursing skills Nursing competency requires accumulation of evidence based knowledge and skills, understanding of cultural diversity and the care provided. During clinical practice I used the ANMC competency standards to reflect on my own practice. Areas that I felt competent in was my providing care in a safely manner and my ability to address the physiological, psychological, physical, emotional and spiritual needs of the patients and significant others. In addition, I continued professional education in order to build up knowledge and skills that will be necessary in coping with the dynamic changes in healthcare. Continuing education ensures that my knowledge is current and level of competency is maintained at a satisfactory level.
- An overall reflection of the day and how you can use your reflection to improve your practice My last placement has proved to be a huge progress. Through observations and clinical practice, I managed to develop my nursing skills both as an independent learner, but also as a member of the health team. Having been assigned patient loads, research medications and or reflecting on how I went, I was able to employ a range of skills that could not have found a better environment to develop in; critical thinking and problem solving, working with others and time management are just few examples. I will continue to do reflective practice to evaluate any clinical incident, positive or negative to attempt to better understand and contemplate the events which have taken place, and the behaviour, emotions and actions of not only myself, but the patient and others involved at that time In doing so, It will increase knowledge surrounding that situation and my professional development.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services

- Reflective Essay Service

- Dissertation Writing Service

- Essay Writing Service
DMCA / Removal Request
If you are the original writer of this reflective essay and no longer wish to have your work published on the NursingAnswers.net website then please:
Our academic writing and marking services can help you!
- Marking Service
- Samples of our Work
- Full Service Portfolio
Related Lectures
Study for free with our range of nursing lectures!
- Drug Classification
- Emergency Care
- Health Observation
- Palliative Care
- Professional Values

Write for Us
Do you have a 2:1 degree or higher in nursing or healthcare?
Study Resources
Free resources to assist you with your nursing studies!
- APA Citation Tool
- Example Nursing Essays
- Example Nursing Assignments
- Example Nursing Case Studies
- Reflective Nursing Essays
- Nursing Literature Reviews
- Free Resources
- Reflective Model Guides
- Nursing and Healthcare Pay 2021
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Lippincott Open Access

Reflective practice in health care and how to reflect effectively
Kiron koshy.
a Brighton and Sussex University Hospital
Christopher Limb
b Western Sussex University Hospitals, Worthing
Buket Gundogan
c UCL Medical School, University College London, London
Katharine Whitehurst
d Royal Devon and Exeter Hospital, UK
Daniyal J. Jafree
Reflective practice is a paper requirement of your career progression in health care. However, if done properly, it can greatly improve your skills as a health care provider. This article provides some structure to reflective practice to allow a health care provider to engage more with reflective practice and get more out of the experience.
Introduction
Reflective practice is something most people first formally encounter at university. This may be reflecting on a patient case, or an elective, or other experience. However, what you may not have considered is that you have been subconsciously reflecting your whole life: thinking about and learning from past experiences to avoid things that did not work and to repeat things that did. For example after tasting a food you do not like, you remember that experience, think about it, and when you next see that same food you know to avoid it. In medicine it is one of the best approaches to convert theoretical knowledge into practice.
As you progress through medical school and into foundation years as a doctor it becomes even more common. It is now expected to provide evidence of your reflections through your training on the ePortfolio and then throughout the rest of your professional life in revalidation. Hence, it is a good idea to get it right from the beginning.
First and foremost the biggest mistake you can make when reflecting is to treat it as a tick box exercise and a waste of time. With a bit of thought reflections can be a very useful tool in learning. Would you remember a generic case from a book? Would hanging all of those facts on a patient you have met make it more memorable? It allows you to recognize your own strengths and weakness, and use this to guide on-going learning. By reflection you will develop your skills in self-directed learning, improve motivation, and improve the quality of care you are able to provide.
What to reflect on
This can be anything.
Most reflections are on things that go wrong. These situations stay in one’s head and force us to begin to think about whether they could have done anything differently. For example:
- Postoperative complications
- Missed diagnosis
- A dissatisfied patient
- Failed procedure
However, reflecting on things that went well can often be more rewarding and be just as useful. It can build confidence and help you to repeat it again on another occasion. For example:
- A well-managed cardiac arrest
- An interesting seminar or conference
- A patient thank you letter
- A difficult but well performed procedure
Stages of reflecting
There are numerous models for reflections, but it is important to understand why you are asking each question and how that will help you to reflect 1 . This an integration of many concepts but the broad process is similar in all models: what happened, why does this matter and what are the next steps? 2
What, where, and who—the situation
Think about the situation in detail: What happened exactly and in what order, where were you at the time and who else was involved? What part did you have to play? What was the final outcome?
How did it make you feel—your emotional state
What was running through your head and how did you feel about it? Be honest with yourself: were you afraid, confused, angry or scared? If you can understand how you were feeling at the time it will help you put together why things happened as they did, and help you to recognize similar situations in the future.
Why did it happen—making sense of the situation
Now you have thought about the situation in greater detail, and probably recognized things that would have otherwise gone unnoticed, think about why things happened as they did. How did the situation, yourself, and others interact at the time. Did the situation go well or was there room for improvement?
Could you have done anything differently—critical review and development of insight
With the help of hindsight how would you have managed the situation differently? Think about what factors you could have influenced: is there anything you could have tried that may have improved the situation, or is there anything you did that was particularly important in the situation? It is easy to remember the things that you did not do and it is often the things that you did well that are forgotten.
What will you do differently in the future—how will this change your practice
This is arguably the most important stage in reflecting. You need to pull together everything you have thought of before to learn, change your own practice, and improve 3 . Do not only think about what you would do differently in that specific situation, but think whether you have thought of any transferable knowledge or skills you can utilize elsewhere. For example: if you reflect on a postprocedural complication do not only think of how you would manage this again but also how you would prevent it happening if you performed the procedure yourself! If you are a part of a well-led cardiac arrest do not think only of what you would do next to help, but also how you would lead an arrest in the future, or even how you would lead a team in any other situation!
Re-enforcement—what happens when you put this into practice
Test your reflections: When comparable situations happen again, do things change as you would expect them to? This is a chance to repeat the reflective cycle to refine and develop your understanding.
How to make the best use of reflective practice
As mentioned previously most people see reflective practice as a tick box exercise, but it does not have to be.
Over the next day take note of any interesting situations that arise. Later in the day try mentally reflecting, following this framework, and if you think any will be particularly useful to you write them down. If you try this for a week you will begin to see similar situations arising and how your reflective practice is positively affecting you.
Remember: you do not always have to learn only from your own experience; learn from others’ mistakes as well. Reflect on situations that you have witnessed to work out why things happened as they did, and how this can influence you.
It can be useful to take these reflections for peer or senior review: others may be able to draw light on things you have not noticed. This can allow you to recognize points for improvement and work on them. This can also be a useful learning opportunity for the other involved!
An example to put this into practice
I was involved in a patient confrontation; the patient was unhappy with her hospital stay and wanted to be discharged home. Unfortunately she required a package of care and so could not be discharged. I explained this and she returned to her bed. I was happy I had explained everything to her and continued with my other jobs.
Who, what, and why
I was involved in a patient confrontation; an elderly patient was unhappy with hospital stay and wanted to be discharged home. She was under our general surgical team for a head injury and observation after a normal CT head. She had been seen on our ward round and told that she was medically fit for discharge but still awaiting social services: her house had been reviewed and deemed unsafe so she was waiting for banisters to be installed. The issue was raised with me by chance as I was doing other things on the ward. I explained this to her and although she remained annoyed I was able to make her understand what the delay was and she returned to her bedside. She did not seek further clarification that day.
How did it make you feel
At the time I felt rushed and frustrated. I had a lot of other work to be done and this was distracting from that. She had already been told she was waiting for social services in the morning. I understood why this was difficult for her but did not think I would be able to do anything to help.
Why did it happen
The morning ward round was quite rushed and so our explanation was limited to telling her we were waiting for social services. I can understand from her point of view this may have meant very little, and so my explanation of what exactly we were doing may have relieved some frustration. Having been waiting up to this point, it is no surprise she continued to be angry but may have been accepting of this plan.
Could you have done anything differently
I think my explanation was very good, and the patient seemed happy with this, although I did not give a rough idea of how long this would take. It may have been useful to have spoken to the sister in charge to ask for what progress had been made to feed back to the patient. Also I did not ask her whether she was happy with this explanation: I may have been able to satisfy her frustration further by answering a few more questions or even recognize any other issues at home that may need addressing before discharge. Although the information given in the ward round was correct, it was not understandable to the patient. If this had all been quickly clarified in the morning, the patient would have been happy throughout the day and not caused a problem later on.
What will you do differently in the future
I think that the route problem in this situation was our explanation on the morning ward round. Furthermore, I am not sure how long such issues take to be addressed. To avoid a similar situation in the future I will speak to the other health care professionals on the ward to get a round idea of how long occupational interventions such as this and other community interventions take to start. This means when future patients are medically fit I can spend a moment in the morning informing them of what needs to be done and how long it may take. Hopefully this will allow me to address patient concerns early to avoid them becoming an issue when it is too late.
Re-enforcement
I will reflect on how future situations similar to this develop, looking for an improvement in the quality of my patient care.
Following a structure helps to focus a reflection: I am sure you will agree the learning points are much clearer from a good reflection!
Conclusions
To summarize, the benefits of reflecting are clear: it may be difficult to do initially, but through practice you will develop your own skills and become a better learner. Many structures are available so choose one what works for you. Reflective practice is an important part of your career progression on paper, but if done well, can greatly improve your skills as a health care provider.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 June 2017
- Login / FREE TRIAL

‘The new government is going back for the future of the NHS’
STEVE FORD, EDITOR
- You are here: Students
Reflecting on health and wellbeing as a student nurse: a personal journey
09 August, 2021
One student nurse’s personal reflection and experience of the theoretical and lay understanding of the factors that contribute to health and wellbeing
This article presents a reflection of my personal health journey since starting university as a student nurse. Focusing on social, physical and psychological aspects of health, it explores the impact of stress and the cessation of exercise on my health and wellbeing. The similarities and differences of lay and theoretical definitions of health are examined, and a personal definition of health is presented. The discussion presents my understanding of health as a student nurse, and analyses how loneliness has impacted on my health and wellbeing during the first two terms of university.
Citation: Power C, Andrewes T (2021) Reflecting on health and wellbeing as a student nurse: a personal journey. Nursing Times [online]; 117: 8, 22-24.
Authors: Caela Power is student nurse, Tanya Andrewes is lecturer in adult nursing; both at Bournemouth University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
Introduction
My experience in the first two terms of university as a student nurse led me to reflect on our understanding of health and wellbeing from a theoretical and lay perspective. A better personal understanding of managing health and wellbeing can support student nurses in their role of helping patients to explore and improve their health.
Theoretical perspectives
Defining health is complex due to the high number of factors that contribute to it. The World Health Organization (1946) defined health as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”. Yet, this definition has been criticised for being limited in its scope, on the basis that there are many other factors to consider beyond disease and infirmity: as discussed below (Warwick-Booth et al, 2012).
Theoretical models are developed and tested by experts through the collection of research evidence (Topolski, 2009), and enable us to understand different dimensions of health. As shown in Box 1, the four main models of health are medical, social, holistic and biopsychosocial, each of which explains the impact of different factors on health and wellbeing (Warwick-Booth et al, 2012).
Box 1. Four theoretical models of health
- Medical – focuses on physiological aspects of health
- Social – takes into account external factors such as those that are environmental, cultural and economic
- Holistic – places the individual at the centre
- Biopsychosocial – features an awareness of the balance between psychological, social, biological and spiritual influences
The medical model focuses on physiological aspects of health. The theory has developed from scientific evidence about the impact of illness on the systems of the body and the impact of medical/surgical interventions as a response to it. The emphasis in the medical model is on diagnosis and treatment from an expert (Warwick-Booth et al, 2012). If a medically defined illness is absent, then health is considered to be good.
The medical model focuses on biomedical influences on an individual’s health alone, without consideration of the social and psychological dimensions. Blaxter (2010) acknowledged that, while medicine has a place for supporting individuals to achieve their maximum physiological health potential, other theoretical models need to be considered if nurses are to support holistic health and wellbeing that takes into account social and psychological dimensions of health.
The social model of health takes a more holistic approach, viewing the individual as a social, complex being. In the social model, external factors such as environmental, cultural and economic influences are considered in terms of their impact on people’s health and wellbeing (Michaelson, 2013). According to the social model, how individuals perceive their life can affect their ability to cope with stresses (Blaxter, 2010). This internal capacity to cope and overcome adversity is known as resilience.
In contrast to both the medical and social models, the holistic and biopsychosocial models focus on the interplay between the psychological, social, biological and spiritual influences on individuals, reinforcing the need for a healthy balance between all these factors to achieve good health. The holistic and biopsychosocial models consider each person as a unique individual, subject to discrete circumstances, experiences and expectations (Lehman et al, 2017).
The holistic model places the individual at the centre, empowering them to enhance their own health and wellbeing through what Blaxter (2010) called alternative therapies. This approach is supported in the UK by a report from the Government Office for Science (GOS), which introduced the Five ways to Wellbeing model; individuals are encouraged to:
- Connect with others;
- Take notice;
- Keep learning;
- Give (GOS, 2008).
All these activities support holistic and/or biopsychosocial health and wellbeing.
Lay perspectives
The lay perspectives on health, some characteristics of which are summarised in Box 2, refer to informal definitions or a general understanding of what good health means, with a recognition that the understanding varies between individuals. Yuill et al (2010) highlighted that lay perspectives are informed by class-based, generational traditions and norms, often based on personal and familial experiences; Kolderup Hervik (2016) suggested that lay perspectives are gendered and contextual.
Box 2. Characteristics of lay perspectives on health
- Informal definitions or an understanding of good health that varies between individuals
- Informed by class-based, generational traditions and norms, and often based on personal and familial experiences
- In the past, dismissed as being ill informed and subjective; now more likely to be seen as valid, even in the absence of an evidence base
Lay perspectives reflect a non-expert view, in which some beliefs and understandings about health and illness exist in the absence of research evidence. They inform people’s experiences of health, their interpretations of the causes and effects of illness, and their responses to it. One such example is the understanding in the Middle Ages that illness was caused by evil spirits, with the ‘treatment’ involving driving out the spirits by torturing the body (Yuill et al, 2010).
In the past, lay knowledge of good health has been dismissed as being ill informed and subjective; however, as it has developed over time, some individuals, including Warwick-Booth et al (2012), have proposed that lay perspectives are valid, even in the absence of an evidence base. This is because individuals have the best insight into their own health and wellbeing, factors that affect it and actions that help to support it.
For some, health is viewed as physical; for others, it is psychological or sociological and/or holistic (Yuill et al, 2010). Blaxter (2010) explored lay perspectives of health among participants from the UK and revealed five key areas of understanding – namely, that being healthy means being not ill and having vitality, physical fitness, social relationships and psychosocial wellbeing.
Similarities
Theoretical and lay perspectives share some similarities, in part because theoretical influences contribute to lay perspectives of health (Entwistle et al, 1998). Medicine has a powerful influence on a person’s perspective of health and the evidence base of the medical model offers a strong and reliable foundation for understanding physiological illness (Blaxter, 2010) – it is easy to understand the positive impact being physically fit has on health. Indeed, if individuals can maintain physical fitness, they can reduce their chances of developing some illnesses, for example, cardiac disease (Warwick-Booth et al, 2012).
The theory underpinning the social model is reflected in lay perspectives that link good health with a good social life, including the ability to maintain relationships, have a secure socioeconomic status and financial stability (GOS, 2008). Some lay beliefs are more holistic, incorporating aspects of the holistic and biopsychosocial models in which health is viewed as a balance between the biological, social and psychological.
Differences
There are some significant differences between theoretical and lay perspectives of health. Firstly, theoretical perspectives are grounded in evidence, whereas it is usual that lay beliefs are unconsciously developed as a result of individual life experiences and relationships, alongside social influences such as the media (Yuill et al, 2010).
Another key difference is that theoretical perspectives do not necessarily change significantly over time but, instead, evolve with the further collection of research data around their use. By contrast, lay perspectives commonly change in time due to individuals being exposed to varied life experiences, alongside factors such as increasing age (Rydstedt et al, 2004). Theoretical perspectives are used to underpin healthcare policies because they are based on evidence, but lay perspectives tend to be more informal (Blaxter, 2010).
A personal definition
My own definition of health is focused on achieving the maximum quality of life, emotionally, spiritually, physically and socially, and feeling a regular sense of contentedness in all four interlinked aspects. I believe this can, or should, be achieved by taking a natural, holistic approach, while also understanding the importance of biomedicine and its place in improving health.
My personal beliefs about health reflect the biopsychosocial model, as I believe in taking a person-centred approach to improving health with the consideration of one’s interlinking psychological, social and physical circumstances. In addition, I believe spirituality plays a vital role in a person’s health and deserves equal focus to physiological aspects.
Since moving far from my family home to study for an adult nursing degree, my health has become increasingly important to me. Moving to a new area was daunting and had many impacts on my health and wellbeing. Initially, I stayed with family friends, life was exciting and I felt positive about my prospects as a university student and a nurse. I was happy, I socialised regularly and had an exercise routine.
As time passed, however, I began to feel the pressures of academic expectation and I socialised less. I lacked motivation to complete academic work, leading to assignments being submitted late. The resulting high levels of stress and anxiety had an impact on my psychological wellbeing: I felt socially isolated, began to feel extremely low in confidence and lonely, and lacked the motivation to exercise. I was also beginning to make unhealthy food choices. All these changes had a negative impact on my physical state.
I was worried I wouldn’t cope with being on placement; conversely, however, the placement provided a sense of purpose and confirmed my passion for nursing, as well as teaching me some coping strategies to enhance my wellbeing.
Dealing with loneliness
Making new friends is a challenging and sometimes difficult experience. I rely on connecting with others – a feature of the Five Ways to Wellbeing model – to help maintain my wellbeing and maximise life enjoyment. However, after starting university I found it difficult to make friends and connect with people, which reduced my self-confidence and created feelings of insecurity. Loneliness had a negative impact on my psychological state and mental health, causing some anxiety and a depressive mood.
Vasileiou et al (2019) have pointed out that loneliness among university students is a recognised problem, with 64% of students admitting to feeling lonely during their studies; loneliness and social isolation has also been closely linked to poor mental health and a reduced sense of wellbeing while studying at university.
The low levels of confidence I had because of loneliness led to depressive feelings and my increasingly unhealthy diet, as well as significantly reducing my physical activity. Despite understanding the situation, I found it extremely difficult to change my mindset and lifestyle. My motivation to study and my potential to enjoy being a nursing student were severely compromised. I recognised the imperative to change, so I was well enough to care for others in my student nursing role.
Once I understood that I was lonely, I worked to overcome this. I explored my spirituality and began to meditate, which helped me to appreciate solitude, and worked to identify how I could improve my health and wellbeing through deep exploration of my feelings/thoughts. Zollars et al (2019) have promoted the use of meditation to reduce stress and increase overall good health and, as my mental health improved, I began to socialise and connect with others.
My self-confidence and mood improved and I exercised again, setting myself challenges; this gave me purpose, a sense of self-fulfilment and self-confidence. My positive experience of physical activity supports Herbert et al’s (2020) finding that it improves the mental health, wellbeing and physiological health of university students.
The Covid-19 pandemic has forced several lockdowns, with significant restrictions on social activities, a factor known to increase mental ill health and reduce wellbeing (Dawson and Golijani-Moghaddam, 2020). I knew lockdown would interfere with the coping techniques I was using to overcome my loneliness, such as exercising with others and social interactions in the workplace. Not only did all social activities stop, I was removed from practical placement. I turned to meditation and lengthy outdoor exercise to cope, which helped preserve my resolve to maintain a healthy mindset and lifestyle.
Although my wellbeing has fluctuated over the past year, my increased self-awareness has led to me to take prompt action at the early signs of decline, such as feeling withdrawn and unsociable, under increasing pressure from academic assignments and having a general sense of unease that I am not managing.
Individuals who engage in wellness programmes experience reduced stress and increased functionality at work (Couch, 2014). My self-led meditation practice reduced my stress and increased my engagement in my university course. It has enabled me to analyse links between my physiological and psychological health, and understand the multifaceted influences on health and wellbeing. The importance of maintaining a balance between physical, psychological, social and spiritual aspects is clear.
Without analysing my situation in the context of theoretical and lay perspectives of health, I could not have developed the self-awareness about my loneliness, or my purposeful actions to overcome it. This understanding has implications for my nursing practice as I can draw on my experience to empathise with my patients and ask them questions to help them analyse what is happening and how it affects their health and wellbeing and what small positive changes they can make to bring improvement.
Analysing theoretical and lay perspectives of health, in the context of my personal experience of health and wellbeing since starting university, has enabled me to explore how the different dimensions of health are fundamentally linked and affect each other. I have realised how complex the meaning of good health is, and that it means different things to different people. As such, I appreciate the importance of understanding what health means for me and for those people I will support as a nurse.
- Theoretical and lay perspectives of health enable analysis of the impact of a range of factors on health and wellbeing
- Developing self-awareness allows individuals to identify factors that have a negative impact on health and wellbeing
- Self-awareness of personal health and wellbeing status can help identify coping strategies
- Personal reflection can help guide student nurses’ understanding of health and wellbeing and how it can be improved in those for whom they care
Related files
210811 reflecting on health and wellbeing as a student nurse – a personal journey.
- Add to Bookmarks
Related articles

‘A strong team requires both introverts and extroverts’
Student editor Ellie Sims shares her experience of navigating through university as an introvert.

‘We need a system to support students whose placements are unsuitable’
Student editor Richard Denton highlights potential problems with placement allocation.

‘The decline in mature student numbers is a critical blow to the healthcare sector’
Soneika Atkinson on making nurse education more attractive and accessible for mature students.

‘Courage and candour aren’t just for patient matters, we must look after ourselves too’
Student editor Lucy Allen on the need to talk about stresses.
Have your say
Sign in or Register a new account to join the discussion.
Home — Essay Samples — Nursing & Health — Nursing — Reflective paper on nursing
Nursing: a Reflective Paper on Challenges Encountered
- Categories: Medicare Nursing Nursing Theory
About this sample

Words: 1727 |
Published: Oct 4, 2018
Words: 1727 | Pages: 3 | 9 min read
Table of contents
General overview, challenges in medical and medication supervision, difficulties in the pharmaceutical knowledge, improvement strategies to tackle these identified challenges.
- Works Cited
- Patient advocate
- Unsupervised clinical practice
- Supervision and mentorship/ preceptorship
- Interaction/ liaison within the team
- Counselling
- Accurate and timely documentation.
Adherence to the policies and procedures
Use of technology, development of proper plans and prioritization.
- Levett-Jones, T., & Bourgeois, S. (2011). The clinical placement: An essential guide for nursing students. Elsevier Health Sciences.
- Ott, J., & Ross, C. (2014). The Role of Supervision in Mediation Errors. The Journal for Nurse Practitioners, 10(2), 74-78.
- Australian Commission on Safety and Quality in Health Care. (2012). National safety and quality health service standards. Retrieved from https://www.safetyandquality.gov.au/sites/default/files/migrated/National-Safety-and-Quality-Health-Service-Standards-second-edition.pdf
- Burke, M. A., & Dufault, M. A. (2018). Building resilience in nursing students: An integrative review. Journal of nursing education, 57(12), 699-706.
- Casey, D., Burke, E., Houghton, C., & O’Donoghue, D. (2018). Physiotherapy students’ perceptions of their readiness to work as newly qualified professionals. Physiotherapy Practice and Research, 39(2), 121-128.
- Dieterly, D. L., & Schafer, J. C. (2016). Nursing students' experiences of bullying and incivility in clinical settings: a qualitative study. Journal of Nursing Education, 55(7), 365-370.
- Hafsteinsdottir, T. B., Beckstrand, R. L., & Bond, A. E. (2019). Transition shock in new graduate nurses: A theoretical yet realistic framework. Nursing Forum, 54(2), 250-256.
- Kelly, J., & Ahern, K. (2018). Nursing student anxiety in simulation settings: A mixed-methods study. Clinical Simulation in Nursing, 19, 34-41.
- National Council of State Boards of Nursing. (2015). Delegation: Concepts and decision-making process. Retrieved from https://www.ncsbn.org/Delegation_consensus_final.pdf

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Prof Ernest (PhD)
Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 587 words
2 pages / 1053 words
1 pages / 460 words
1 pages / 821 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online

Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Nursing
Gagnon, K., & Sabus, C. (2015). Professionalism in a Digital Age: Opportunities and Considerations for Using Social Media in Health Care. Physical Therapy, 95(3), 406–414.Nayak, S. G. (2018). Time Management in Nursing -- Hour [...]
A good nurse possesses a unique blend of qualities that extend far beyond medical knowledge and technical skills. In this essay, we will explore the essential qualities of a good nurse and how they significantly contribute to [...]
American Nurses Association. (2021). Nursing: Scope and Standards of Practice (4th ed.). Silver Spring, MD: American Nurses Association.Nightingale, F. (1860). Notes on Nursing: What It Is, and What It Is Not. Harrison and [...]
When driving around Miami, FL there are a copious number of billboards that I see advertising for people to become nurses throughout the city. When doing research, I’ve only come across a couple of news articles, advertising on [...]
In today's ever-evolving healthcare landscape, nurses play a crucial role in ensuring the well-being and health of individuals across the globe. From providing hands-on care to advocating for patient rights, nurses are the [...]
We are living in a global diversity where different cultures, religions, traditions, and spiritual beliefs play important parts in making our lives. As in the United States, the growing diversity has been seen for decades and [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Nursing Reflective Essay: Example Outline and Guide
Are you a nursing student? Then, you will definitely have an assignment to compose a nursing reflective essay. This task might be quite tough and challenging. But don’t stress out! Our professionals are willing to assist you.
First and foremost, let’s figure out what’s reflective writing. In a few words, such essays are based on your experiences, feelings, views on something, etc. For nursing students, reflective papers help to practice their skills and expand their knowledge.
In case you are wondering how to master nursing essay writing, you are on the right page. This article will provide tips that will help you write an outstanding student nurse reflection: examples of what to include in it, what to avoid, and what reflection ideas to use.
- ✒️ Analytical Essay
- ✒️ Reflective Essay
⭕ Outline and Draft
- ⭕ Revise and Edit
- ❓ Reflecting
- ❗ Essential Parts
- ⚠️ Mistakes to Avoid
- 💡 Additional Tips
🔗 References
🆚 analytical vs. reflective nursing essay.
Basically, nursing essays can be divided into different types.
The most common ones are analytical and reflective essays. These two styles are the most suitable for nursing papers. They either argue a particular perspective (analytical) or base the narration on previous experiences (reflective).
This focus is what differentiates one type of essay from another, as both analytical and reflective nursing essays can be quite similar stylistically.
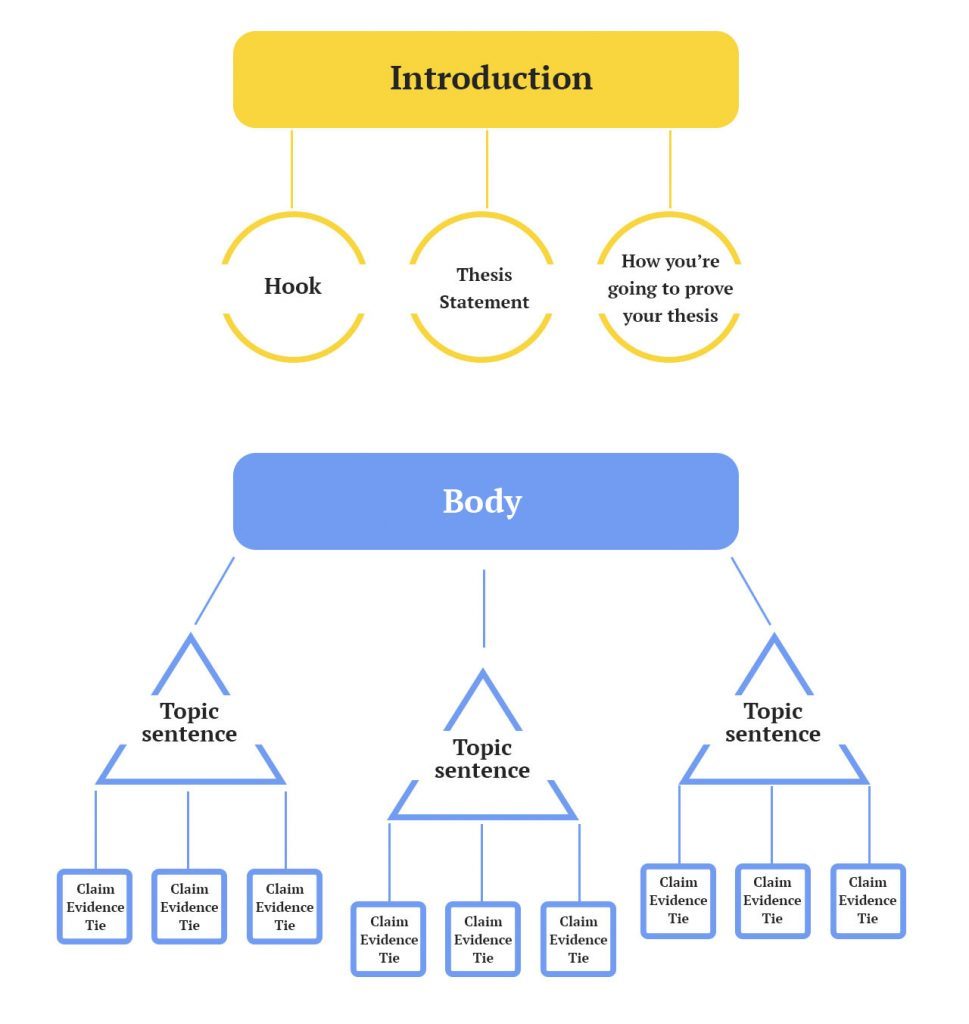
✒️ Nursing: Analytical Essay
Speaking of structural parts, the basic outline for an analytical essay on nursing can look like this:
- Introduction. The introduction is your first paragraph, which usually consists of two parts. First, you have to capture the reader’s attention. Then, you aim to create a clear thesis statement to demonstrate your position.
- Providing evidence. Each of your arguments should have at least one piece of evidence. If you have more to share, it’s even better. However, you have to keep the word count in mind. So, make strong points to keep your essay concise.
- Practical use of evidence. There’s no point in providing evidence that wouldn’t have any real use in practice.
- The judgment of evidence. Here, you’ll demonstrate your critical thinking abilities. Prove that your evidence is indeed strong and valid.
- Conclusion. First, summarize what’s been said in your essay. Then give your own opinion on it.
✒️ Nursing: Reflective Essay
A reflective nursing essay usually consists of the following elements:
- Introduction. It explains the whole purpose of the essay and tells readers what to expect. The most crucial part of the introduction is the thesis statement. Usually, it’s the last sentence of the intro. Your thesis should be concise and clearly reflect your position.
- Description of the situation. Since a reflective essay is based on previous experiences, describing those events is the core part of the paper. Again, this section should contain only the most important and valuable details.
- Sharing of personal feelings. The section demonstrates how you react to situations and how you’re able to gain control over those emotions.
- Evaluation of experience. This part is a summary of what skills and knowledge did you get from experience.
- Reflection and opportunities for learning. The part is closely connected to the previous one. While previously you were just giving a summary, here you’ll describe what this situation made you understand. Also, elaborate on your future path of self-development.
- Conclusion. This one is simple. Just summarize all the information you discussed in your essay.
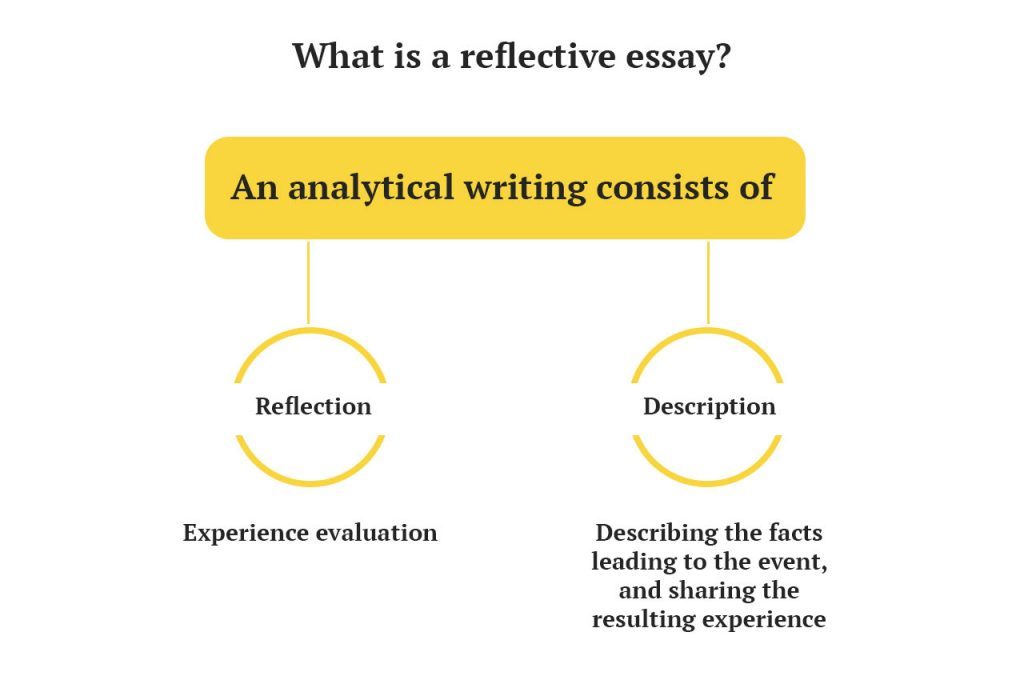
We’ll now tell you about the writing preparation process.
✍️ Reflective Nursing Essay Writing Guide
Creating a nursing reflection essay is a long and challenging journey that requires total concentration on a task. That’s why it is essential to turn your “study mode” on and devote yourself to the essay writing process.
No doubts, you might get too overwhelmed by the amount of work you have to get done. But don’t worry! We know how to avoid anxiety. Just follow out tips and write an outstanding nursing reflection paper!
⭕ Remove the Distractions
Yes, it’s as simple as that. Ironically, most of the major problems usually happen at this initial stage. So, you just need to know how to overcome them right away.
- Turn all the gadgets off (yes, even your cell phone).
- Make sure to get rid of anything around you that could potentially catch your attention and distract you from writing.
- Get earplugs if necessary.
Then, get in your “ study mood .”
- Go through all your pre-game rituals — visit the bathroom, eat, drink, meditate, etc.
- Get fun out of the way. Dedicate some time for yourself so that you won’t have the temptation later.
- Prepare the essentials — open your notebook or create a Word document and make sure you have all the necessary notes and resources ready.
After you create perfect conditions, make another significant step to start writing. Research is an essential part of any academic work, even if you’re telling your personal story.
Here’s where you can start your search for brilliant ideas:
- your college library;
- your course readings;
- scholar.google.com ;
- online nursing journal articles;
And no matter what topic you’re going to discuss, don’t forget that most nursing essays use a similar pattern:
You still need to remember what we talked about in the very beginning—your story has to be unique. Use these questions as a framework, and fill the essay with unique content. If you’ve answered most of these questions, you’re halfway to your excellent grade.
No wonder outlining is a vital part of the essay writing process. It helps to reach the logical flow of ideas and organize all the arguments and examples in the right order. Also, you can easily edit a well-developed structure if something seems wrong. It’s always better to change the part of an outline rather than rewriting the entire paragraph.
Your reflective essay outline should look approximately like this:
- Point 1 (evidence, examples, other supportive details)
- Point 2 (evidence, examples, other supportive details)
- Point 3 (evidence, examples, other supportive details)
- Restatement of thesis statement
- Final memorable statement
After creating an outline, it would be a great idea to write the rough draft of your nursing reflective essay. This step will help you to avoid all the possible mistakes before submitting the paper.
⭕ Proofread and Revise
Polishing your essay is the last but not the least step of the writing process. So, take it seriously and don’t waste your chance to submit a flawless work. Revise your rough draft, make sure your ideas are coherent and supporting evidence is logical, and then create a final version of the essay.
We created the checklist of what you should take into consideration while revising your nursing reflective essay. Don’t hesitate to use it!
- make sure you meet the writing style requirements;
- check whether all in-text citations have the corresponding reference list entry;
- make sure you followed the proper structure (introduction, three body paragraphs, conclusion);
- get rid of all grammar and punctuation mistakes;
- double-check whether your ideas are clear and the supporting details are logical.
📝 Reflective Essay: Nursing School Application
Although people say there is a lack of nurses in the United States and a high demand for good specialists with a stellar education, it is not that easy to pass a nursing school admission.
What’s the reason for this?
It seems like nursing schools are looking for outstanding students, who have made a firm decision to become a nurse and will contribute to the community of the school and the field of healthcare in general.

You need to showcase this capability in your nursing application essay, where you reflect on your goals or experience. In the following sections, we’ll explain how to write it.
❓ Reflecting on Your Goals
Here’s a question you should ask yourself.
“Why do I want to become a nurse?”
As curious as it may sound, some students do not have that answer. If you are one of them, you might want to reconsider your decision.
Being a nurse is stressful. It requires dedication, a strong philosophy, and stable mental health. And if you’re unsure about your choice — try to make up your mind as soon as possible. Getting a nursing education is a serious step in your life. So, be confident about your choice not to regret it later. The video below will give you an idea of what a day in the life of a nurse is like.
If your intentions are firm and you’re sure about your career goals, then we’re here to help you cope with all those “Why I chose nursing” essays.
- Know where to begin with your nursing essay. A good idea would be to find several nursing essay examples first. You will see that many students do not try to be creative. The same boring stories can be found in numerous essays. And it seems that those stories are taken from the same articles. This does not sound impressive at all. Repeating familiar stories is quite a critical mistake, and you should avoid it. We’ll talk more about common errors in nursing essay writing later, so keep on reading. Look through nursing paper examples and try to make your essay on nursing career different. Make it stand out from the crowd.
- Base your essay on a true story. Think about any situation from your clinical placement, which your reflective nursing essay will be based on. Describe what happened, who was involved in the case, why it impressed you, etc. By doing this, you’ll demonstrate a complete understanding of the situation. Your essay will show that you’re able to comprehend what’s happening and make the right decisions, even in critical cases. These are skills that every nurse should have.
- Write a personal essay. Now, proceed to describe your feelings. Follow these three questions: How did you feel in that situation? Why? What were your actions, actions of other personnel? Be sure to tell how much you care about what happened. It’s a great way to show how sympathetic you can be.
- Evaluate the event. Another stage of writing the reflective nursing essay is an evaluation of your experience. Why do you think it is a valuable experience for you? How did it contribute to your personal and professional development? Self-development is among the most important aspects of professionalism for a nurse. And you must not forget to show that you have this capability.
- Choose the right focus. It’s common for students to concentrate on a story that deals with the nursing profession. This is a strategy that you too can follow when writing your nursing essay. Make sure that the narrative focuses on you instead of other nurses or patients. It is your essay, so be sure to write about your personal experience. You have to tell the reader about all the lessons you have learned and explain how it helped you come up with a decision to become a nurse.
- Allow yourself to fantasize. There’s another critical subtopic you should cover in your nursing essay. Tell what other actions you could take and whether you would repeat them if you are to face the same situation.
Next, we’re going to talk about the parts that make up a well-written nursing essay. So, stay with us!
❗ Essential Application Essay Parts
You probably know that competition in nursing schools is especially severe compared to other educational institutions.
Your paper should be outstanding if you want to be accepted. Below, you’ll find a list of the most significant parts of any nursing admission essay. These parts contain all relevant info that the admission officers want to know about an applicant.
- Is there someone who influenced your decision?
- Was there an event that had an impact on your choice of a career?
- Do you know what particular kind of nursing you want to do?
These are essential questions to answer in the nursing school essay. You do double-duty by responding to these questions. Not only will you explain how you decided to become a nurse, but you’ll also make sure that your intentions are truly firm.
- Show what you’re worth. You need to tell about your experience in the nursing field. This is a job where you need at least some background experience. If you have never seen a syringe in your life, your nursing essay will hardly convince anybody. So, make a personal statement. And make it strong.
- Prove that you’re ready. You need to show that you are prepared to be a nurse and ready for all nursing hardships. Nursing is emotionally and physically exhausting. That’s why you need to demonstrate the best of your personal and professional values. Prove to the admission counselors that you are capable of completing the nursing job.
⚠️ Nursing Reflective Essay: Mistakes to Avoid
As we have mentioned before, the competition is going to be tough. It is not easy to enter a nursing school even with the best scores and a high GPA. So, your essay should be of the highest quality!
One of the best ways to ensure your works’ flawlessness is to learn the most common mistakes and avoid them in your paper. Below, you will find the list of the most critical errors for a nursing application essay. Investigate them here:
- Not including your own nursing story. Without a real-life story, a nursing application essay seems very weak. Listing personal qualities that a nurse should possess is not enough. Tell a story that will show the admission officers how helpful you were while providing care to somebody.
- Not sharing your experiences. Your nursing application essay will undoubtedly lose a couple of points if you do not mention some nursing-related experiences. A strong intention to become a nurse is great, of course. However, if you have no idea what nursing is all about, your essay will not be convincing.
- Not providing sufficient reasons to support your intentions. It is ridiculous to remind about that, but some students still fail to mention why they want to enter a certain school. Make sure to avoid this mistake in your nursing application essay.
- Plagiarizing. Plagiarism can cause you to fail your paper or be rejected by the schools you’re applying for. More importantly, plagiarizing isn’t ethical. To avoid any potential issues, make sure to cite all your sources and provide an authentic and unique story.
- Repeating yourself. This problem is widespread in the introduction and conclusion. If you need to connect the first and the last paragraphs, don’t just rewrite the info from the introduction into a conclusion. Rather synthesize your thoughts and finish your essay dynamically.
💡 Nursing Reflective Essay: Additional Tips
We’ve come to the point where you can go ahead and start writing your nursing essay. But there’s one more thing…
The process of writing a nursing essay has its own tips and tricks. So, we simply couldn’t let you go without sharing a few of them.
Hopefully, you’ll find these nursing school essay tips useful:
- Always start working on your student nurse essay with studying assignment instructions. It is the best way to find out what exactly will be assessed in your paper.
- Do not hesitate to ask your tutors for help and advice. Whether you believe it or not, but they do not want you to fail student nurse assignments and essays.
- To prepare a good student nurse essay, you will have to find and read quite a lot of nursing literature. Mind that it usually takes a lot of time. So, start researching early.
- Student nurse essays have a standard structure. Thus, make sure you have all the necessary paragraphs. Do not forget about specific terminology that should be used in your works. Also, remember about the proper formatting of your paper (e.g., MLA, Chicago, or APA format).
- How to summarize an article without plagiarizing ? Keep your text balanced. When looking through your draft, pay attention not only to the logic of your narration. Also, check how much of the paper consists of your own thoughts and what percentage is a review of other people’s work.
With these tips in mind, it’s now your time to shine!
Nursing Reflective Essay Topics
- Strengths and weaknesses of nursing professionals.
- Analyze the methods clinical nurses use to deal with moral distress.
- Examine the types of errors in nursing.
- Describe the role of technology in nursing care.
- The role of the nursing professional in patient advocacy.
- Peculiarities and importance of nursing interventions in the pre-op period.
- The crucial role of communication in nursing.
- Is MSN course worth studying?
- Examine the importance of cultural competency in nursing practice.
- What is the best nursing recruitment strategy and why?
- The critical role of QSEN competencies in becoming a better nurse.
- Collaboration and communication issues in nursing practice .
- Personal integrity and accountability in nursing practice.
- Describe the aims and key concepts of nursing care delivery model.
- Analyze the basic nursing theories.
- Discuss the occupational health and safety risks of practicing nurses.
- How to reduce the rate of workplace burnouts in nursing profession.
- Advantages and disadvantages of becoming a clinical nurse leader.
- Analyze the main reasons for nursing shortage.
- Describe the challenges of family nurse practitioner and the ways to overcome them.
- The importance of nurses providing care for elderly at home .
- Examine the elements that the nurses should take into account to improve the care of diabetic patients.
- Why knowledge and application of psychological theories are vital for nursing practice.
- Why is it necessary to integrate cultural competence in nursing practice?
- Do Christian values play an important role in nursing practice?
- Discuss the significance of evidence-based practice to nursing.
- Explain how a nurse can use a personal digital assistant to enhance performance.
- The challenges of language barrier and cultural diversity in nursing practice.
- Cultural sensitive approach in nursing and health care.
- Describe ethical and moral dilemmas of nursing.
- Why strategic management is important to a trained nurse.
- Discuss the issues of combining a nursing job and Jewish traditions.
- The issue of aging of the nursing population.
- Analyze the peculiarities of Cuban-American culture and its influence on nursing practice.
- The role of nurses in healthcare delivery.
- The issue of applying theory to practice to manage stress in nursing.
- Describe the advantages and disadvantages of different leadership styles in nursing.
- Examine the specifics of women’s nursing.
- The leadership role of nurses in the healthcare system.
- Explain how a nurse can be a cultural broker of the community.
- Why do people choose to become nurses?
- The types and benefits of technologies used in nursing profession.
- Analyze why a mandatory overtime in nursing profession is a controversial solution.
- Describe the reasons of burnout in nursing profession.
- How to resolve the nursing shortage problem.
- Discuss the effective strategies to improve nursing ratios.
- Psychological effects of COVID-19 that result in hospital nursing shortage.
- Compare and analyze the specifics of the community virtual ward model with the inpatient nursing care model.
- The importance of clear vision to nursing leaders.
- Discuss whether the use of social media can violate ethical nursing practices.
Writing a nursing essay doesn’t have to seem so difficult anymore. So, go forth and do it. Make it flawless because it’s your very first step on the way to success.
Also, don’t hesitate to share your thoughts! Did we miss something? Want to add more helpful info? Write about it in the comments below!
Further reading:
- How to Write an Expository Essay in Simple Steps
- Essay on Dengue Fever: How to Write + Free Examples
- Objective Essay Writing: How to Write, Topics and Examples
- French Essay Writing: How-to Guide and Examples
🤔 Reflective Essay in Nursing FAQ
A good essay of this kind is a reflection of personal feelings, impressions, and motivation. It should be based on a real-life example. Describe an unusual situation you’ve faced during the clinical placement program. Analyze the emotions it provoked, what could have been improved, etc.
Before you start writing, focus on your personal impressions provoked by a certain event or topic. Then put them down and organize cohesively. Do not forget about an appropriate introduction and a memorable conclusion.
If you are to write a good application essay to progress your career as a nurse, start with formulating your personal motivation to pursue this occupation. Draft the arguments and relevant examples, and then distill the right wording to make your paper persuasive and impressive.
It is a good idea to read some examples of reflective essay conclusions, but make sure that your own version is personal and sincere. A reflective essay is all about your own thoughts and perceptions, so you may simply paraphrase and summarize them.
- Ten Terrific Tips for New Nurses Dealing with Difficult Patients
- Volunteer Nursing Abroad
- Ten Ways How to Get in and Gain Experience in Nursing
- Critical Thinking and Writing for Nursing Students
- Learning Opportunities in Adolescent Nursing
- Clinical Nursing Resources
- The NHS Constitution for England
- The National League for Nursing Core Values
- Reflective Writing: About Gibbs Reflective Cycle
- Nursing Reflection Essays
- Share to Facebook
- Share to Twitter
- Share to LinkedIn
- Share to email
![nurse reflective essay examples Remembering an Event Essay: Examples and Guidelines [Free]](https://custom-writing.org/blog/wp-content/uploads/2020/12/businessman-touching-eyeglasses-is-using-digital-tablet-284x153.jpg)
Throughout our life, we meet plenty of people and participate in various events. If some of them are just regular, the other people or occasions play a critical role in our fates. Your life-changing experience might become a perfect ground for creating a remembering essay.
![nurse reflective essay examples Environment vs. Development Essay: Tips & Topics [2024]](https://custom-writing.org/blog/wp-content/uploads/2020/12/storage-tanks-284x153.jpg)
Environment vs. development is a multifaceted present days’ dilemma. On the one hand, environmental problems are increasing year after year. We have more polluted areas on our planet, more polluted rivers, fewer trees that produce oxygen. On the other hand, can we stop development and progress in various fields? Is...

What does an essay look like? At a glance, the answer is obvious. An essay looks like a mere piece of paper (one page or several pages) with an organized text. It’s generally divided into five paragraphs, though there may be more. The essential essay structure includes: Yet, will this...

Some students find writing literary analysis papers rather daunting. Yet, an English class cannot go without this kind of work. By the way, writing literary analysis essays is not that complicated as it seems at a glance. On the contrary, this work may be fascinating, and you have a chance...

These days, leadership and ability to work in a team are the skills that everybody should possess. It is impossible to cope with a large educational or work project alone. However, it can also be challenging to collaborate in a team. You might want to elaborate on importance and difficulties...

Racial profiling is not uncommon. It’s incredibly offensive and unfair behavior that causes most of the protests in support of people of color. It occurs when people are suspected of committing a crime based on their skin color or ethnicity. Unfortunately, most people are unaware that racial profiling is an everyday...

Without a doubt, a natural disaster essay is a tough paper to write. To begin with, when people encounter a disaster risk, it’s a tragedy. Emergency situations can affect hundreds, thousands, and millions of people. These are the crises and events that change people’s lives drastically. So, disaster and emergency...

“You are not only responsible for what you say, but also for what you do not say”Martin Luther There are a lot of other good quotations that can serve as a good beginning for your essay on responsibility and provide good ideas for writing.

Exemplification essays, which are also called illustration essays, are considered one of the easiest papers to write. However, even the easiest tasks require some experience and practice. So, if you are not experienced enough in writing exemplification essays, you will face certain challenges.

You push the snooze button once again and finally open your eyes. It is already 8:50, and your classes start at 9. “I’m going to be late again!”— you think, already in full panic mode. In a minute, you rush out the door half-dressed, swallowing your sandwich on the go. ...

An essay about Harriet Tubman is to focus on the biography and accomplishments of a famous American abolitionist and political activist of the 19th century. Harriet Tubman was born into slavery, escaped it herself, and helped others escape it. She changed many jobs throughout her lifetime, being a housekeeper, a...

What is a documented essay and what is the purpose of it? It is a type of academic writing where the author develops an opinion relying on secondary resources. A documented essay can be assigned in school or college. You should incorporate arguments and facts from outside sources into the...
You’re awesome! Thanks for these nursing reflective essay writing tips! Thanks! Thanks! Thanks!
Thanks for your nursing reflective essay guide. It really helped me cope with my reflective essay in nursing.

IMAGES
VIDEO
COMMENTS
This essay aims to critically reflect on an encounter with a service user in a health care setting. The Gibbs' Reflective Cycle will be used as this is a popular model of reflection. Reflection is associated with learning from experience. It is viewed as an important approach for professionals who embrace lifelong learning (Jasper, 2013).
For example, in most nursing reflective essays. Whichever the case, readily available information expands on each model to make it easier to write a reflection essay on a specific aspect of nursing education or practice. Read the assignment rubric and instructions to understand the specific model. If it is unclear, ask for clarification from ...
This example of a reflective essay is presented in association with Price, B and Harrington, A (2013) Critical Thinking and Writing for Nursing Students, London, Learning Matters. Readers are introduced to the process of critical and reflective thinking and the translation of these into
15 Best Reflective Essay Topics: Nursing. Empathy and support in nursing. Communication with patients is one of a nurse's most important tasks. Nursing is the art of caring for the patient during illness. The key is not to cause harm but to help afterward. Time management in nursing.
Nursing Reflection Essay Tips and Examples. Writing a nursing reflective essay requires careful consideration and thoughtful analysis. Here are some tips to help you craft an impactful and meaningful reflection essay as a BSN nursing student. Additionally, we will provide examples to illustrate how these tips can be applied.
Ce n t re f o r A ca d e mi c De ve l o p me n t Using Gibbs: Example of reflective writing in a healthcare assignment. Description. I n a placement during my second year when I was working on a surgical ward, I was working under t he supervision of my ment or, caring f or a sevent y-t wo year old gent leman, Mr K han (pseudonym), who had ...
EXAMPLE ESSAYS FOR Critical Thinking and Writing for Nursing Students Bob Price and Anne Harrington CONTENTS: Example analytical essay - page 1 Example reflective essay - page 11 EXAMPLE ANALYTICAL ESSAY This example of an analytical essay is presented in association with Price, B and Harrington, A (2010)
1920927077. Sample Reflective journal. The following are extracts from a Reflective journal written for the course, Reflective Nursing Practice 1 by a first year student, Chrissy Poulos: ALOUR COMMENTSExcerpt from Wee. IDefining nursing is a tough one. I am not su.
Nursing Reflective Essay Examples. Reading well-written nursing reflective essay samples can be a helpful way to improve your own writing. Analyzing these essays' structure, language, and content, you can better understand what makes a strong reflective essay. Here are two examples to guide you: Example 1: Reflecting on a Challenging Patient ...
Students need guidance from instructors in reflective writing. For example, nurse educators do need to help students identify topics to reflect on (Kennison, 2012). Examples of types of reflective writing include the following: free writing, essays, personal narratives, and journal entries.
Reflective Essay on Teamwork. Last modified: 20th Oct 2021. This essay will critically reflect on the process of teamwork, change management and leadership; all issues pertinent to the role of the SCPHN. Barr and Dowding (2008) assert the necessity for leaders to critically reflect effectively, in order to raise their awareness and effect ...
Simple introductions are there to keep your readers' curiosity. 2. Make It Personal for Your Readers. Another important thing to remember is that when you write your reflective nursing essay, make sure to make it personal. This is one way of getting your readers to sympathize or to empathize with you.
Introduction. This essay aims to critically reflect on an experience where a patient was encountered during clinical placement. The real name of the patient in this reflective essay has been changed for confidentiality reasons as it is the patient's right (Kentucky Board of Nursing, 2018).
The "what nursing means to me" reflective essay is something often asked of students entering an accelerated nursing program, a master's level course for nursing, or some other specialized program. Unlike a nursing school letter of intent or nursing school application cover letter, the "what nursing means to me" reflective essay gives you a chance to state and defend an argument ...
Nursing Associates must be reflective practitioners (NMC, 2018b). I will use Gibb's reflective cycle (Gibbs, 1988), which has 6 stages - Description, Feelings, Evaluation, Analysis, Conclusion and Action Plan - to structure this essay.
Communication is a fundamental element in nursing practice. This element can possibly determine patients' satisfaction and even the outcomes of their treatment (Lotfi et al., 2019). The situation described in the paper will exemplify the potential role of communication, which is why it will serve as a Gibbs Reflective Cycle nursing example.
How to write a reflection essay in Nursing. Nursing is an incredibly challenging and rewarding career choice. In this article, we will explore the basics of writing a reflective essay, which is a type of writing that helps you reflect on your experiences as a nurse and consider what it has taught you about yourself.We will provide tips on how to structure the essay, outline key points, and ...
Nurturing Leadership: 4 Key Strategies for Reflective Practice in Nursing 5 min read • February, 21 2024 Nursing leaders play a pivotal role in guiding change to enhance patient care and improve professional practice environments. Reflective practice stands out as a cornerstone for effective leadership, allowing nursing professionals to ...
Clinical Placement Reflection 2. Communication with others within the clinical setting - this may include staff members, patients and relatives. During my placement I was in a stroke unit. Being on this unit, I worked with many clients who suffered from post-stoke symptoms and many had difficulty with speech. The experience helped me learn ...
Introduction. Reflective practice is something most people first formally encounter at university. This may be reflecting on a patient case, or an elective, or other experience. However, what you may not have considered is that you have been subconsciously reflecting your whole life: thinking about and learning from past experiences to avoid ...
Abstract. This article presents a reflection of my personal health journey since starting university as a student nurse. Focusing on social, physical and psychological aspects of health, it explores the impact of stress and the cessation of exercise on my health and wellbeing. The similarities and differences of lay and theoretical definitions ...
Nursing: a Reflective Paper on Challenges Encountered. Nursing is an exciting profession, but with the levels of interest, there is still a sheer level of challenges that are encountered in the nursing environment. Thus, by writing a nursing reflective essay, I would identify the strategies that are applicable and variable to be applied when ...
Point 1 (evidence, examples, other supportive details) Point 2 (evidence, examples, other supportive details) Point 3 (evidence, examples, other supportive details) Conclusion. Restatement of thesis statement. Final memorable statement. After creating an outline, it would be a great idea to write the rough draft of your nursing reflective essay.