Advertisement

A type of vitamin B3 might treat chronic pain related to inflammation
Chronic pain can outlast inflammation, the usual driver of pain in the body – a study in mice suggests a vitamin supplement could help relieve it
By Grace Wade
8 November 2023

The mitochondria in certain sensory neurons could be linked to chronic pain
nobeastsofierce Science / Alamy
A type of vitamin B3, called nicotinamide riboside, alleviates persistent pain in mice, suggesting that it may potentially treat chronic pain in humans as well.
Inflammation – the body’s first line of defence against injury and pathogens – is a main driver of pain. Yet, some people continue to experience pain even after inflammation has resolved.
To understand why, Niels Eijkelkamp at Utrecht University in the Netherlands and his colleagues analysed inflammation’s impact on mitochondria , the powerhouses of cells. Previous research has linked chronic pain to dysfunctional mitochondria, particularly those in specialised nerve cells, called sensory neurons, which detect changes in the environment.
The researchers injected a substance that triggers inflammation into the hind paws of 15 mice. They then measured the amount of oxygen consumed by mitochondria in the sensory neurons of the animals, which indicates mitochondrial function. They found that a week later, after inflammation had resolved, mitochondria consumed significantly more oxygen than they had before the injection, suggesting inflammation caused lasting changes to their function. Further experiments linked these mitochondrial changes to greater pain sensitivity in the rodents even after their inflammation resolved.
Eight healthy habits that could slow your rate of ageing
The researchers then analysed the molecular byproducts of chemical reactions, called metabolites, inside the mitochondria of the animals. They compared these to the mitochondrial metabolites in mice that had not experienced induced inflammation. The team found that after inflammation had resolved, mice had lower than expected levels of nicotinamide riboside in the mitochondria of their sensory neurons. This is a type of vitamin B3 critical for mitochondrial function.
Sign up to our Health Check newsletter
Get the most essential health and fitness news in your inbox every Saturday.
So, about a week after inducing inflammation in a separate group of 12 mice, Eijkelkamp and his team gave half of them a high dose of nicotinamide riboside – 500 milligrams per kilogram of body weight. By comparison, the recommended daily amount of vitamin B3 for most adults is between 14 and 16 milligrams . They then assessed the animals’ sensitivity to pain by measuring how quickly they pulled their paw away from heat. Mice that hadn’t received nicotinamide riboside retracted their paw twice as fast, on average, as those that did, suggesting the supplement alleviates pain.
Together these findings indicate two things: first, that inflammation can impair mitochondrial function in sensory neurons and that these impairments increase the risk of chronic pain, even after inflammation has resolved. Second, that taking nicotinamide riboside supplements may help treat this chronic pain by restoring mitochondrial function.
However, people with chronic pain shouldn’t rush to take these supplements. “[This research] is still in rodents. How does it translate to humans? We really have to see that first,” says Eijkelkamp. Clinical trials may show nicotinamide riboside has no effect or even unintended consequences, he says.
Even if these findings do translate to humans, they probably only apply to certain types of chronic pain, such as that of chronic inflammatory diseases , says Eijkelkamp. For instance, more than 20 per cent of people with rheumatoid arthritis – a chronic condition characterised by persistent joint inflammation – continue to have pain even with low levels of inflammation. As such, it would make sense to test these findings in that demographic first.
Journal reference:
Cell Reports Medicine DOI: 10.1016/j.xcrm.2023.101265
- cell biology /
- immune system /
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
More from New Scientist
Explore the latest news, articles and features
Asthma treated in mice using offshoot of CAR T-cell cancer therapy
Ultrasound therapy could treat lung condition linked to heart failure.
Subscriber-only
Autoimmune conditions linked to reactivated X chromosome genes
The new drugs preventing allergic reactions to peanuts and other foods, popular articles.
Trending New Scientist articles

Nicotinamide Riboside Supplement Linked to Reduced Biomarkers of Alzheimer’s in the Brain
Summary: Nicotinamide riboside (NR), a naturally occurring dietary supplement can enter the brain and alter the metabolism of biological pathways associated with Alzheimer’s disease.
Source: University of Delaware
For the first time, a researcher at the University of Delaware College of Health Sciences in collaboration with a team at the National Institute on Aging, a division of the National Institutes of Health, has determined that the naturally occurring dietary supplement, nicotinamide riboside (NR), can enter the brain.
The discovery was made by Christopher Martens, assistant professor of kinesiology and applied physiology and director of the Delaware Center for Cognitive Aging Research, and Dr. Dimitrios Kapogiannis, a senior investigator at the National Institute on Aging.
The finding is significant because it supports the idea that NR, upon reaching the brain, can alter the metabolism of relevant biological pathways involved in neurodegenerative diseases like Alzheimer’s.
Their work, supported by an NIH grant, and in part by the Intramural Research Program of the NIH National Institute on Aging, was recently published in the journal Aging Cell .
Upon consumption, NR is readily converted into nicotinamide adenine dinucleotide (NAD+), which is critical to cellular repair and the repair of damaged DNA.
“NAD+ is gradually lost as we get older or develop chronic diseases. Loss of NAD+ is linked to obesity and other negative lifestyle habits like smoking,” Martens said. “Because more NAD+ is needed to counteract those negative consequences, it’s more likely to be depleted in the face of negative lifestyle habits.”
Martens has been studying the compound since he was a postdoctoral fellow at the University of Colorado Boulder. In an initial study, he found that levels of NAD+ could be boosted in the blood if people ingested NR, but it was not clear if it could reach other tissues in the body.
“We had some preliminary signs of efficacy, including lower blood pressure in people who had high blood pressure to begin with,” he said. “But until now, it was unknown whether NR reached targeted organs like the brain to have a real therapeutic effect.”
Measuring the level of NAD+ in the brain in humans is challenging. There are emerging techniques involving MRI, but these only provide an indirect measure and are costly and difficult to perform. Instead, Martens and colleagues measured NAD+ directly in tiny particles called extracellular vesicles that originated from neurons and ended up in the blood.
These extracellular vesicles can provide cutting-edge blood-based biomarkers for brain disorders and serve as a “liquid biopsy” of neurons, giving researchers a rare look at what’s inside them.
“Each vesicle has a unique molecular signature on its surface, including proteins that give you clues about its origin,” Martens said. “In our case, we selected vesicles that carry markers that are characteristic of neurons, and so we have confidence that the NAD+ we measured in them reflects what happens in the neurons, and by extension the brain.”
Using samples from their first initial clinical trial, the researchers determined, first, that NAD+ levels went up in these vesicles after six weeks.
“When NAD+ goes up in these vesicles, we see an association with some of the biomarkers of neurodegenerative disease,” Martens said. “Particularly, in people where we saw an increase in NAD+, we also saw changes in biomarkers like amyloid beta and tau, which are both related to Alzheimer’s disease,” Martens said.
Martens and Kapogiannis also found a correlation between these neurodegenerative biomarkers and change in NAD+.
“If NAD+ went up a lot, there was typically a larger change in some of the disease biomarkers,” Martens said. “That tells us the NAD+ is not only getting into the brain but it’s likely also having some effect on its metabolism and multiple interrelated pathways.”
Some of these blood-based biomarkers could be used down the road to determine if NAD+ depletion is a cause of Alzheimer’s and other neurodegenerative diseases. It is even possible that these types of tests could become more accessible to the population for more routine testing.
Martens is leading a 12-week study involving NR in older adults with mild cognitive impairment. The study is supported by the Delaware Center for Cognitive Aging Research and the National Institute on Aging and is actively seeking more participants.

Through the study, Martens seeks to determine whether increased consumption of NR has an even larger effect in people with cognitive impairment.
“They’re coming in with cognitive deficits, and as a result, are more likely to have an accumulation of some of these biomarkers in their brain, so there’s a chance we’ll see bigger reductions in these biomarkers because they have more of them in their cells,” Martens said.
Nearly all drugs on the market for patients with Alzheimer’s have only a modest effect on the symptoms but do not significantly stop the underlying progression of the disease.
“In our ongoing trial, we’re measuring markers of cognitive function and other things related to functional independence and quality of life, but we’re also hoping to gain some insight on the underlying disease process,” Martens said. “We’re hoping that the people who take the NR might have preserved function.”
After proving its efficacy, Martens and Kapogiannis will test whether increased use of NR improves cognition, and ultimately, whether it can be used to slow neurodegenerative disease progression.
“We were among the first to do a chronic dosing study in humans, and as a result, we’ve been at the forefront of this field for a few years,” Martens said. “Now, we’re at a turning point, where we can start to determine whether NAD+ increases in other tissues as well, and that’s likely where the more important signal will be in terms of resolving disease.”
The Delaware Center for Cognitive Aging Research relies on the support of individuals and organizations to accelerate interdisciplinary research, education and clinical training in Alzheimer’s disease and related disorders. If you are interested in learning more about the Center or how you can support this important research, please contact Doug Kleintop .
About this Alzheimer’s disease research news
Author: Marina Affo Source: University of Delaware Contact: Marina Affo – University of Delaware Image: The image is in the public domain
Original Research: Open access. “ Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin ” by Christopher Martens et al. Aging Cell
Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin
Declining nicotinamide adenine dinucleotide (NAD + ) concentration in the brain during aging contributes to metabolic and cellular dysfunction and is implicated in the pathogenesis of aging-associated neurological disorders.
Experimental therapies aimed at boosting brain NAD + levels normalize several neurodegenerative phenotypes in animal models, motivating their clinical translation.
Dietary intake of NAD + precursors, such as nicotinamide riboside (NR), is a safe and effective avenue for augmenting NAD + levels in peripheral tissues in humans, yet evidence supporting their ability to raise NAD + levels in the brain or engage neurodegenerative disease pathways is lacking.
Here, we studied biomarkers in plasma extracellular vesicles enriched for neuronal origin (NEVs) from 22 healthy older adults who participated in a randomized, placebo-controlled crossover trial (NCT02921659) of oral NR supplementation (500 mg, 2x /day, 6 weeks).
We demonstrate that oral NR supplementation increases NAD + levels in NEVs and decreases NEV levels of Aβ42, pJNK, and pERK1/2 (kinases involved in insulin resistance and neuroinflammatory pathways).
In addition, changes in NAD(H) correlated with changes in canonical insulin–Akt signaling proteins and changes in pERK1/2 and pJNK.
These findings support the ability of orally administered NR to augment neuronal NAD + levels and modify biomarkers related to neurodegenerative pathology in humans. Furthermore, NEVs offer a new blood-based window into monitoring the physiologic response of NR in the brain.
Pet Ownership Boosts Men’s Empathy Towards Animals

Viagra Shows Promise in Boosting Brain Blood Flow for Dementia Prevention

Intense Exercise May Sabotage Weight Loss Efforts

Pandemic Reveals Unexpected Resilience in Isolated Seniors
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Emerging role of nicotinamide riboside in health and diseases.

1. Introduction
2. nr and nad + biosynthesis, 3. synthesis of nr, 3.1. biosynthesis of nr, 3.2. chemical synthesis of nr, 3.3. chemo-enzymatic synthesis of nr, 4. nr in health and diseases, 4.1. neuroinflammation, 4.2. fibrosis, 5. nr and covid-19, 6. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Azzini, E.; Raguzzini, A.; Polito, A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021 , 22 , 9694. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021 , 1498 , 9–28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Holubiec, P.; Leonczyk, M.; Staszewski, F.; Lazarczyk, A.; Jaworek, A.K.; Rojas-Pelc, A. Pathophysiology and clinical management of pellagra—A review. Folia Med. Cracov. 2021 , 61 , 125–137. [ Google Scholar ]
- Trapani, S.; Rubino, C.; Indolfi, G.; Lionetti, P. A Narrative Review on Pediatric Scurvy: The Last Twenty Years. Nutrients 2022 , 14 , 684. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dewett, D.; Lam-Kamath, K.; Poupault, C.; Khurana, H.; Rister, J. Mechanisms of vitamin A metabolism and deficiency in the mammalian and fly visual system. Dev. Biol. 2021 , 476 , 68–78. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mladenka, P.; Macakova, K.; Kujovska Krcmova, L.; Javorska, L.; Mrstna, K.; Carazo, A.; Protti, M.; Remiao, F.; Novakova, L. OEMONOM Researchers and Collaborators, Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022 , 80 , 677–698. [ Google Scholar ] [ CrossRef ]
- Souganidis, E. Nobel laureates in the history of the vitamins. Ann. Nutr. Metab. 2012 , 61 , 265–269. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022 , 327 , 2326–2333. [ Google Scholar ]
- Viswanathan, M.; Treiman, K.A.; Kish-Doto, J.; Middleton, J.C.; Coker-Schwimmer, E.J.; Nicholson, W.K. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017 , 317 , 190–203. [ Google Scholar ] [ CrossRef ]
- Annweiler, C.; Dursun, E.; Feron, F.; Gezen-Ak, D.; Kalueff, A.V.; Littlejohns, T.; Llewellyn, D.J.; Millet, P.; Scott, T.; Tucker, K.L.; et al. ‘Vitamin D and cognition in older adults’: Updated international recommendations. J. Intern. Med. 2015 , 277 , 45–57. [ Google Scholar ] [ CrossRef ]
- Granato, D.; Carocho, M.; Barros, L.; Zabetakis, I.; Mocan, A.; Tsoupras, A.; Cruz, A.G.; Pimentel, T.C. Implementation of Sustainable Development Goals in the dairy sector: Perspectives on the use of agro-industrial side-streams to design functional foods. Trends Food Sci. Technol. 2022 , 124 , 128–139. [ Google Scholar ] [ CrossRef ]
- Lordan, R.; Rando, H.M.; Consortium, C.-R.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. Msystems 2021 , 6 , e00122-21. [ Google Scholar ] [ CrossRef ]
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic—Implications for consumers and regulatory oversight. PharmaNutrition 2021 , 18 , 100282. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 2007 , 129 , 473–484. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008 , 28 , 115–130. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016 , 7 , 12948. [ Google Scholar ] [ CrossRef ]
- Canto, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012 , 15 , 838–847. [ Google Scholar ] [ CrossRef ]
- Vaur, P.; Brugg, B.; Mericskay, M.; Li, Z.; Schmidt, M.S.; Vivien, D.; Orset, C.; Jacotot, E.; Brenner, C.; Duplus, E. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 2017 , 31 , 5440–5452. [ Google Scholar ] [ CrossRef ]
- Roboon, J.; Hattori, T.; Ishii, H.; Takarada-Iemata, M.; Nguyen, D.T.; Heer, C.D.; O’Meally, D.; Brenner, C.; Yamamoto, Y.; Okamoto, H.; et al. Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD. J. Neurochem. 2021 , 158 , 311–327. [ Google Scholar ] [ CrossRef ]
- Trammell, S.A.; Weidemann, B.J.; Chadda, A.; Yorek, M.S.; Holmes, A.; Coppey, L.J.; Obrosov, A.; Kardon, R.H.; Yorek, M.A.; Brenner, C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep. 2016 , 6 , 26933. [ Google Scholar ] [ CrossRef ]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stodkilde-Jorgensen, H.; Moller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018 , 108 , 343–353. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Altamirano, F.; Szweda, P.A.; Elnwasany, A.; Lee, D.I.; Yoo, H.; Kass, D.A.; Szweda, L.I.; et al. NAD(+) Repletion Reverses Heart Failure with Preserved Ejection Fraction. Circ. Res. 2021 , 128 , 1629–1641. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Heer, C.D.; Sanderson, D.J.; Voth, L.S.; Alhammad, Y.M.O.; Schmidt, M.S.; Trammell, S.A.J.; Perlman, S.; Cohen, M.S.; Fehr, A.R.; Brenner, C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J. Biol. Chem. 2020 , 295 , 17986–17996. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grunewald, M.E.; Chen, Y.; Kuny, C.; Maejima, T.; Lease, R.; Ferraris, D.; Aikawa, M.; Sullivan, C.S.; Perlman, S.; Fehr, A.R. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog. 2019 , 15 , e1007756. [ Google Scholar ] [ CrossRef ]
- Bryan, S.; Baregzay, B.; Spicer, D.; Singal, P.K.; Khaper, N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol. 2013 , 91 , 22–30. [ Google Scholar ] [ CrossRef ]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021 , 49 , 101195. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Curtin, N.; Banyai, K.; Thaventhiran, J.; Le Quesne, J.; Helyes, Z.; Bai, P. Repositioning PARP inhibitors for SARS-CoV-2 infection(COVID-19); a new multi-pronged therapy for acute respiratory distress syndrome? Br. J. Pharmacol. 2020 , 177 , 3635–3645. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zheng, M.; Schultz, M.B.; Sinclair, D.A. NAD(+) in COVID-19 and viral infections. Trends Immunol. 2022 , 43 , 283–295. [ Google Scholar ] [ CrossRef ]
- Curry, A.; White, D.; Cen, Y. Small Molecule Regulators Targeting NAD(+) Biosynthetic Enzymes. Curr. Med. Chem. 2022 , 29 , 1718–1738. [ Google Scholar ] [ CrossRef ]
- Yang, Y.; Zhang, N.; Zhang, G.; Sauve, A.A. NRH salvage and conversion to NAD(+) requires NRH kinase activity by adenosine kinase. Nat. Metab. 2020 , 2 , 364–379. [ Google Scholar ] [ CrossRef ]
- Kurnasov, O.; Goral, V.; Colabroy, K.; Gerdes, S.; Anantha, S.; Osterman, A.; Begley, T.P. NAD biosynthesis: Identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 2003 , 10 , 1195–1204. [ Google Scholar ] [ CrossRef ]
- Lin, H. Nicotinamide adenine dinucleotide: Beyond a redox coenzyme. Org. Biomol. Chem. 2007 , 5 , 2541–2554. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016 , 23 , 1127–1139. [ Google Scholar ] [ CrossRef ]
- Dietrich, L.S.; Fuller, L.; Yero, I.L.; Martinez, L. Nicotinamide mononucleotide pyrophosphorylase activity in animal tissues. J. Biol. Chem. 1966 , 241 , 188–191. [ Google Scholar ] [ CrossRef ]
- Preiss, J.; Handler, P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J. Biol. Chem. 1958 , 233 , 488–492. [ Google Scholar ] [ CrossRef ]
- Bieganowski, P.; Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 2004 , 117 , 495–502. [ Google Scholar ] [ CrossRef ]
- Tempel, W.; Rabeh, W.M.; Bogan, K.L.; Belenky, P.; Wojcik, M.; Seidle, H.F.; Nedyalkova, L.; Yang, T.; Sauve, A.A.; Park, H.W.; et al. Nicotinamide riboside kinase structures reveal new pathways to NAD+. PLoS Biol. 2007 , 5 , e263. [ Google Scholar ] [ CrossRef ]
- Fletcher, R.S.; Ratajczak, J.; Doig, C.L.; Oakey, L.A.; Callingham, R.; Da Silva Xavier, G.; Garten, A.; Elhassan, Y.S.; Redpath, P.; Migaud, M.E.; et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 2017 , 6 , 819–832. [ Google Scholar ] [ CrossRef ]
- Yang, T.; Chan, N.Y.; Sauve, A.A. Syntheses of nicotinamide riboside and derivatives: Effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J. Med. Chem. 2007 , 50 , 6458–6461. [ Google Scholar ] [ CrossRef ]
- Ryu, D.; Zhang, H.; Ropelle, E.R.; Sorrentino, V.; Mazala, D.A.; Mouchiroud, L.; Marshall, P.L.; Campbell, M.D.; Ali, A.S.; Knowels, G.M.; et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 2016 , 8 , 361ra139. [ Google Scholar ] [ CrossRef ]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD(+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016 , 17 , 679–690. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, L.E.; Sinclair, D.A. Restoring stem cells—All you need is NAD(.). Cell Res. 2016 , 26 , 971–972. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Canto, C.; Auwerx, J. Targeting sirtuin 1 to improve metabolism: All you need is NAD(+)? Pharmacol. Rev. 2012 , 64 , 166–187. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020 , 31 , 564–579.e7. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients 2020 , 12 , 1616. [ Google Scholar ] [ CrossRef ]
- Giroud-Gerbetant, J.; Joffraud, M.; Giner, M.P.; Cercillieux, A.; Bartova, S.; Makarov, M.V.; Zapata-Perez, R.; Sanchez-Garcia, J.L.; Houtkooper, R.H.; Migaud, M.E.; et al. A reduced form of nicotinamide riboside defines a new path for NAD(+) biosynthesis and acts as an orally bioavailable NAD(+) precursor. Mol. Metab. 2019 , 30 , 192–202. [ Google Scholar ] [ CrossRef ]
- Long, D.J., 2nd; Jaiswal, A.K. NRH: Quinone oxidoreductase2 (NQO2). Chem. Biol. Interact. 2000 , 129 , 99–112. [ Google Scholar ] [ CrossRef ]
- Lu, S.P.; Lin, S.J. Phosphate-responsive signaling pathway is a novel component of NAD+ metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 2011 , 286 , 14271–14281. [ Google Scholar ] [ CrossRef ]
- Belenky, P.; Stebbins, R.; Bogan, K.L.; Evans, C.R.; Brenner, C. Nrt1 and Tna1-independent export of NAD+ precursor vitamins promotes NAD+ homeostasis and allows engineering of vitamin production. PLoS ONE 2011 , 6 , e19710. [ Google Scholar ]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Dolle, C.; Niere, M.; Yakimov, A.; Redpath, P.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J. Biol. Chem. 2015 , 290 , 27124–27137. [ Google Scholar ] [ CrossRef ]
- Haynes, L.J.; Hughes, N.A.; Kenner, G.W.; Todd, A. Codehydrogenases. 2. A Synthesis of Nicotinamide Nucleotide. J. Chem. Soc. 1957 , 3727–3732. [ Google Scholar ] [ CrossRef ]
- Jeck, R.; Woenckhaus, C. Simple methods for preparing nicotinamide mononucleotide and related analogs. Methods Enzymol. 1980 , 66 , 62–70. [ Google Scholar ] [ PubMed ]
- Tanimori, S.; Ohta, T.; Kirihata, M. An efficient chemical synthesis of nicotinamide riboside (NAR) and analogues. Bioorg. Med. Chem. Lett. 2002 , 12 , 1135–1137. [ Google Scholar ] [ CrossRef ]
- Franchetti, P.; Pasqualini, M.; Petrelli, R.; Ricciutelli, M.; Vita, P.; Cappellacci, L. Stereoselective synthesis of nicotinamide beta-riboside and nucleoside analogs. Bioorg. Med. Chem. Lett. 2004 , 14 , 4655–4658. [ Google Scholar ] [ CrossRef ]
- Zhang, N.; Sauve, A.A. Synthesis of beta-Nicotinamide Riboside Using an Efficient Two-Step Methodology. Curr. Protoc. Nucleic Acid Chem. 2017 , 71 , 14.14.1–14.14.9. [ Google Scholar ] [ CrossRef ]
- Szczepankiewicz, B.; KOPPETSCH, K.; Perni, R.B. Preparation and Use of Crystalline Beta-d-Nicotinamide Riboside. Google Patents PCT/IB2015/054181, 10 December 2015. [ Google Scholar ]
- Makarov, M.V.; Migaud, M.E. Syntheses and chemical properties of beta-nicotinamide riboside and its analogues and derivatives. Beilstein J. Org. Chem. 2019 , 15 , 401–430. [ Google Scholar ] [ CrossRef ]
- Tran, A.; Yokose, R.; Cen, Y. Chemo-enzymatic synthesis of isotopically labeled nicotinamide riboside. Org. Biomol. Chem. 2018 , 16 , 3662–3671. [ Google Scholar ] [ CrossRef ]
- Cercillieux, A.; Ciarlo, E.; Canto, C. Balancing NAD(+) deficits with nicotinamide riboside: Therapeutic possibilities and limitations. Cell Mol. Life Sci. 2022 , 79 , 463. [ Google Scholar ] [ CrossRef ]
- Reiten, O.K.; Wilvang, M.A.; Mitchell, S.J.; Hu, Z.; Fang, E.F. Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech. Ageing Dev. 2021 , 199 , 111567. [ Google Scholar ] [ CrossRef ]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013 , 75 , 685–705. [ Google Scholar ] [ CrossRef ]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016 , 352 , 1436–1443. [ Google Scholar ] [ CrossRef ]
- Gong, B.; Pan, Y.; Vempati, P.; Zhao, W.; Knable, L.; Ho, L.; Wang, J.; Sastre, M.; Ono, K.; Sauve, A.A.; et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 2013 , 34 , 1581–1588. [ Google Scholar ] [ CrossRef ]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD(+) Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016 , 24 , 566–581. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Roberts, E.L.; Newton, R.P.; Axford, A.T. Plasma purine nucleoside phosphorylase in cancer patients. Clin. Chim. Acta 2004 , 344 , 109–114. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. USA 2021 , 118 , e2011226118. [ Google Scholar ] [ CrossRef ]
- Hou, Y.; Lautrup, S.; Cordonnier, S.; Wang, Y.; Croteau, D.L.; Zavala, E.; Zhang, Y.; Moritoh, K.; O’Connell, J.F.; Baptiste, B.A.; et al. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA 2018 , 115 , E1876–E1885. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joshi, U.; Evans, J.E.; Pearson, A.; Saltiel, N.; Cseresznye, A.; Darcey, T.; Ojo, J.; Keegan, A.P.; Oberlin, S.; Mouzon, B.; et al. Targeting sirtuin activity with nicotinamide riboside reduces neuroinflammation in a GWI mouse model. Neurotoxicology 2020 , 79 , 84–94. [ Google Scholar ] [ CrossRef ]
- Harlan, B.A.; Killoy, K.M.; Pehar, M.; Liu, L.; Auwerx, J.; Vargas, M.R. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp. Neurol. 2020 , 327 , 113219. [ Google Scholar ] [ CrossRef ]
- Obrador, E.; Salvador, R.; Marchio, P.; Lopez-Blanch, R.; Jihad-Jebbar, A.; Rivera, P.; Valles, S.L.; Banacloche, S.; Alcacer, J.; Colomer, N.; et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1(G93A) Mice. Mol. Neurobiol. 2021 , 58 , 1345–1371. [ Google Scholar ] [ CrossRef ]
- Lee, H.J.; Yang, S.J. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. Int. J. Mol. Sci. 2019 , 20 , 4196. [ Google Scholar ] [ CrossRef ]
- de Castro, J.M.; Stein, D.J.; Medeiros, H.R.; de Oliveira, C.; Torres, I.L.S. Nicotinamide Riboside Neutralizes Hypothalamic Inflammation and Increases Weight Loss without Altering Muscle Mass in Obese Rats under Calorie Restriction: A Preliminary Investigation. Front. Nutr. 2021 , 8 , 648893. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, C.C.; Chen, W.X.; Wang, J.; Xia, M.; Jia, Z.C.; Guo, C.; Tang, X.Q.; Li, M.X.; Yin, Y.; Liu, X.; et al. Nicotinamide riboside rescues angiotensin II-induced cerebral small vessel disease in mice. CNS Neurosci. Ther. 2020 , 26 , 438–447. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mateuszuk, L.; Campagna, R.; Kutryb-Zajac, B.; Kus, K.; Slominska, E.M.; Smolenski, R.T.; Chlopicki, S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020 , 178 , 114019. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, R.; Zhou, Y.; Wang, S.; Pang, N.; Huang, Y.; Ye, M.; Wan, T.; Qiu, Y.; Pei, L.; Jiang, X.; et al. Nicotinamide riboside protects against liver fibrosis induced by CCl4 via regulating the acetylation of Smads signaling pathway. Life Sci. 2019 , 225 , 20–28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, H.J.; Yang, S.J. Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes. Nutr. Res. Pract. 2019 , 13 , 3–10. [ Google Scholar ] [ CrossRef ]
- Pham, T.X.; Bae, M.; Kim, M.B.; Lee, Y.; Hu, S.; Kang, H.; Park, Y.K.; Lee, J.Y. Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019 , 1865 , 2451–2463. [ Google Scholar ] [ CrossRef ]
- Zhou, B.; Wang, D.D.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020 , 130 , 6054–6063. [ Google Scholar ] [ CrossRef ]
- Zhang, M.; Weng, H.; Zheng, J. NAD(+) repletion inhibits the endothelial-to-mesenchymal transition induced by TGF-beta in endothelial cells through improving mitochondrial unfolded protein response. Int. J. Biochem. Cell Biol. 2019 , 117 , 105635. [ Google Scholar ] [ CrossRef ]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019 , 28 , 1717–1728.e6. [ Google Scholar ] [ CrossRef ]
- Romani, M.; Sorrentino, V.; Oh, C.M.; Li, H.; de Lima, T.I.; Zhang, H.; Shong, M.; Auwerx, J. NAD(+) boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Rep. 2021 , 34 , 108660. [ Google Scholar ] [ CrossRef ]
- Igarashi, M.; Miura, M.; Williams, E.; Jaksch, F.; Kadowaki, T.; Yamauchi, T.; Guarente, L. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell 2019 , 18 , e12935. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, B.; Dan, X.; Hou, Y.; Lee, J.H.; Wechter, N.; Krishnamurthy, S.; Kimura, R.; Babbar, M.; Demarest, T.; McDevitt, R.; et al. NAD(+) supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell 2021 , 20 , e13329. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dolopikou, C.F.; Kourtzidis, I.A.; Margaritelis, N.V.; Vrabas, I.S.; Koidou, I.; Kyparos, A.; Theodorou, A.A.; Paschalis, V.; Nikolaidis, M.G. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: A double-blind cross-over study. Eur. J. Nutr. 2020 , 59 , 505–515. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sun, X.; Cao, B.; Naval-Sanchez, M.; Pham, T.; Sun, Y.B.Y.; Williams, B.; Heazlewood, S.Y.; Deshpande, N.; Li, J.; Kraus, F.; et al. Nicotinamide riboside attenuates age-associated metabolic and functional changes in hematopoietic stem cells. Nat. Commun. 2021 , 12 , 2665. [ Google Scholar ] [ CrossRef ]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell Mol. Med. 2010 , 14 , 457–487. [ Google Scholar ] [ CrossRef ]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019 , 19 , 477–489. [ Google Scholar ] [ CrossRef ]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome—A Key Player in Antiviral Responses. Front. Immunol. 2020 , 11 , 211. [ Google Scholar ] [ CrossRef ]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017 , 23 , 899–916. [ Google Scholar ] [ CrossRef ]
- Kang, S.; Kim, C.H.; Jung, H.; Kim, E.; Song, H.T.; Lee, J.E. Agmatine ameliorates type 2 diabetes induced-Alzheimer’s disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacology 2017 , 113 Pt A , 467–479. [ Google Scholar ] [ CrossRef ]
- Mawson, A.R.; Croft, A.M. Gulf War Illness: Unifying Hypothesis for a Continuing Health Problem. Int. J. Environ. Res. Public Health 2019 , 16 , 111. [ Google Scholar ] [ CrossRef ]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017 , 377 , 162–172. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019 , 30 , 630–655. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005 , 115 , 209–218. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021 , 9 , 730176. [ Google Scholar ] [ CrossRef ]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C.; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008 , 47 , 1363–1370. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Han, X.; Bao, X.; Lou, Q.; Xie, X.; Zhang, M.; Zhou, S.; Guo, H.; Jiang, G.; Shi, Q. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 2019 , 7 , e7568. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, M.B.; Pham, T.X.; vanLuling, M.; Kostour, V.; Kang, H.; Corvino, O.; Jang, H.; Odell, W.; Bae, M.; Park, Y.K.; et al. Nicotinamide riboside supplementation exerts an anti-obesity effect and prevents inflammation and fibrosis in white adipose tissue of female diet-induced obesity mice. J. Nutr. Biochem. 2022 , 107 , 109058. [ Google Scholar ] [ CrossRef ]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007 , 13 , 952–961. [ Google Scholar ] [ CrossRef ]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and over Population. Front. Public Health 2017 , 5 , 335. [ Google Scholar ] [ CrossRef ]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018 , 15 , 505–522. [ Google Scholar ] [ CrossRef ]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017 , 21 , 7–13. [ Google Scholar ] [ CrossRef ]
- Murray, M.F.; Nghiem, M.; Srinivasan, A. HIV infection decreases intracellular nicotinamide adenine dinucleotide [NAD]. Biochem. Biophys. Res. Commun. 1995 , 212 , 126–131. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tran, T.; Pencina, K.M.; Schultz, M.B.; Li, Z.; Ghattas, C.; Lau, J.; Sinclair, D.A.; Montano, M. Reduced Levels of NAD in Skeletal Muscle and Increased Physiologic Frailty Are Associated with Viral Coinfection in Asymptomatic Middle-Aged Adults. J. Acquir. Immune Defic. Syndr. 2022 , 89 (Suppl. S1), S15–S22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021 , 12 , 1618. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020 , 181 , 1036–1045.e9. [ Google Scholar ] [ CrossRef ]
- Luscher, B.; Verheirstraeten, M.; Krieg, S.; Korn, P. Intracellular mono-ADP-ribosyltransferases at the host-virus interphase. Cell Mol. Life Sci. 2022 , 79 , 288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Altay, O.; Arif, M.; Li, X.; Yang, H.; Aydin, M.; Alkurt, G.; Kim, W.; Akyol, D.; Zhang, C.; Dinler-Doganay, G.; et al. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv. Sci. 2021 , 8 , e2101222. [ Google Scholar ] [ CrossRef ]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020 , 395 , 514–523. [ Google Scholar ] [ CrossRef ]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020 , 368 , 779–782. [ Google Scholar ] [ CrossRef ]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020 , 584 , 154–156. [ Google Scholar ] [ CrossRef ]
- Hung, I.F.; Lung, K.C.; Tso, E.Y.; Liu, R.; Chung, T.W.; Chu, M.Y.; Ng, Y.Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020 , 395 , 1695–1704. [ Google Scholar ] [ CrossRef ]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021 , 102 , 501–508. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed. Forces India 2020 , 76 , 370–376. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, W.F.; Stephen, P.; Theriault, J.F.; Wang, R.; Lin, S.X. Novel Coronavirus Polymerase and Nucleotidyl-Transferase Structures: Potential to Target New Outbreaks. J. Phys. Chem. Lett. 2020 , 11 , 4430–4435. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Esam, Z.; Akhavan, M.; Lotfi, M.; Bekhradnia, A. Molecular docking and dynamics studies of Nicotinamide Riboside as a potential multi-target nutraceutical against SARS-CoV-2 entry, replication, and transcription: A new insight. J. Mol. Struct. 2022 , 1247 , 131394. [ Google Scholar ] [ CrossRef ]
- Arora, M.K.; Grover, P.; Asdaq, S.M.B.; Mehta, L.; Tomar, R.; Imran, M.; Pathak, A.; Jangra, A.; Sahoo, J.; Alamri, A.S.; et al. Potential role of nicotinamide analogues against SARS-COV-2 target proteins. Saudi J. Biol. Sci. 2021 , 28 , 7567–7574. [ Google Scholar ] [ CrossRef ]
| Condition | Route of Administration | Mechanism of Action | Ref. |
|---|---|---|---|
| Neuroinflammation | Intracerebro ventricular | suppresses CD38-mediated neuroinflammation by increasing NAD levels and suppressing NF-κB in mice | [ ] |
| Oral (supplemented with drinking water) (12 mM) for 5 months | reduces NLRP3 inflammasome expression and proinflammatory cytokines in AD mouse model | [ ] | |
| Oral (supplemented with drinking water) (12 mM) for 6 months | suppresses neuroinflammation in AD/Polβ mice by reducing the levels of proinflammatory cytokines IL-α, TNFα, MCP-1, IL-1β, MIP-1α and increasing the levels of anti-inflammatory cytokine IL-10 | [ ] | |
| Oral (supplemented with diet; 100 µg/kg daily) for 2 months | reduces inflammation in Gulf War Illness mice by increasing the deacetylation of NF-κB p65 subunit and PGC-1α | [ ] | |
| Oral (supplemented with diet at 400 mg/kg); Oral (185 mg/kg) | decreases neuroinflammatory markers in amyotrophic lateral sclerosis (ALS) mice models | [ , ] | |
| Oral, via stomach gavage (400 mg/kg) for 6 weeks | reduces the level of amyloid-β precursor protein and inflammatory markers NLRP3, ASC, and caspase-1 in AD mice models | [ ] | |
| Oral (400 mg/kg) for 4 weeks; Oral (supplemented with food 300 mg/kg) for 28 days | reversed the increased levels of TNFα in the hypothalamus of obese rats and cerebral small vessel disease mice | [ , ] | |
| 100 µM for 24 h | suppressed endothelial inflammation by reducing ICAM1 and von Willebrand factor expression in IL-1β and TNFα-stimulated human aortic endothelial cells | [ ] | |
| Liver Fibrosis | Oral, via stomach gavage (400 mg/kg) for 8 weeks | reversed the development of CCl -induced liver fibrosis in C57BL/6 mice by reducing TGF-β and serum ALT levels | [ ] |
| 100 µM to 10 mM for 24 h | reduced the levels of proinflammatory cytokines TNFα and IL-6, and upregulated the levels of the anti-inflammatory molecule, adiponectin, in AML12 mouse hepatocytes | [ ] | |
| Oral (400 mg/kg daily) for 20 weeks | Inhibits activation of HSCs by reducing the levels of fibrotic markers α-smooth muscle actin, collagen 1α1, and collagen 6α1 | [ ] | |
| Heart failure and cardiac fibrosis | Oral (2 × 250–1500 mg daily) for 9 days | reduced the expression of proinflammatory IL-6 in PBMCs of individuals with Stage D heart failure | [ ] |
| Oral (400 mg/kg) for 6–8 weeks | improves the expression of prohibitin to suppress the progression of TGF-1β-induced endothelial-to-mesenchymal transition in cardiac fibrosis | [ ] | |
| Oral (supplemented with diet at 400 mg/kg) for 4 weeks | improved mitochondrial function in heart failure with preserved ejection fraction mice by repleting NAD levels | [ ] | |
| Aging | Oral (1 g daily) for 21 days | reduces circulatory levels of inflammatory cytokines IL-2, IL-5, IL-6, TNFα and augments skeletal muscle NAD without altering its mitochondrial bioenergetics in humans | [ ] |
| Oral (400 mg/kg) for 8 weeks | reduces amyloid aggregation, improves mitochondrial membrane potential and function in mammalian cells | [ ] | |
| Oral (supplemented with drinking water at 50 mg/kg) for 6 weeks | rejuvenates intestinal stem cells in aged mice by activating SIRT1 and mTORC1 | [ ] | |
| Oral (supplemented with drinking water at 12 mM) for 2 months | restores mitochondrial function and homeostasis in ataxia telangiectasia mice models | [ ] | |
| Oral (500 mg) | improved physical performance and decreased oxidative stress in old individuals | [ ] | |
| Oral (400 mg/kg) for 8 weeks | induces change in hematopoietic stem cells composition of aged mice towards a more youthful state by regulating the levels of mitophagy-promoting genes’ transcription | [ ] |
| Treatment Regimen | Description | Type | Status | Clinical Trial |
|---|---|---|---|---|
| 1 g of NR or placebo orally every morning for 14 days | to investigate whether NR supplementation can attenuate the severity of SARS-CoV-2 infections in elderly patients | randomized double-blinded case–control trial | Unknown | NCT04407390 |
| 250 mg NR capsules administered twice daily for 10 days | treatment with NR in COVID-19 patients for renal protection | prospective, double-blind, placebo-controlled clinical interventional trial | Active, not recruiting | NCT04818216 |
| 2000 mg NR in the form of capsules daily | to examine recovery in people with persistent cognitive and physical symptoms after COVID-19 illness | Double-blinded, randomized, parallel-group, placebo-controlled design | Recruiting | NCT04809974 |
| hydroxychloroquine (standard therapy) + dietary supplement consisting of serine, L-carnitine tartrate, N-acetylcysteine, and NR | metabolic cofactor supplementation and hydroxychloroquine combination in COVID-19 patients | parallel-group, randomized, and open-label study | Recruiting | NCT04573153 |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Sharma, C.; Donu, D.; Cen, Y. Emerging Role of Nicotinamide Riboside in Health and Diseases. Nutrients 2022 , 14 , 3889. https://doi.org/10.3390/nu14193889
Sharma C, Donu D, Cen Y. Emerging Role of Nicotinamide Riboside in Health and Diseases. Nutrients . 2022; 14(19):3889. https://doi.org/10.3390/nu14193889
Sharma, Chiranjeev, Dickson Donu, and Yana Cen. 2022. "Emerging Role of Nicotinamide Riboside in Health and Diseases" Nutrients 14, no. 19: 3889. https://doi.org/10.3390/nu14193889
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD + in healthy middle-aged and older adults
Christopher r. martens.
1 Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO USA
Blair A. Denman
Melissa r. mazzo, michael l. armstrong.
2 Department of Pharmaceutical Sciences, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Denver, CO USA
Nichole Reisdorph
Matthew b. mcqueen, michel chonchol.
3 Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Denver, CO USA
Douglas R. Seals
Associated data.
The data that support the findings of this study are available from the corresponding author upon reasonable request. This trial is registered on ClinicalTrials.gov under the identifier {"type":"clinical-trial","attrs":{"text":"NCT02921659","term_id":"NCT02921659"}} NCT02921659 .
Nicotinamide adenine dinucleotide (NAD + ) has emerged as a critical co-substrate for enzymes involved in the beneficial effects of regular calorie restriction on healthspan. As such, the use of NAD + precursors to augment NAD + bioavailability has been proposed as a strategy for improving cardiovascular and other physiological functions with aging in humans. Here we provide the evidence in a 2 × 6-week randomized, double-blind, placebo-controlled, crossover clinical trial that chronic supplementation with the NAD + precursor vitamin, nicotinamide riboside (NR), is well tolerated and effectively stimulates NAD + metabolism in healthy middle-aged and older adults. Our results also provide initial insight into the effects of chronic NR supplementation on physiological function in humans, and suggest that, in particular, future clinical trials should further assess the potential benefits of NR for reducing blood pressure and arterial stiffness in this group.
Declining NAD + levels have been linked to aging-associated pathologies. Here the authors present results of a double-blind, randomized crossover trial on 30 healthy middle-aged individuals to show that nicotinamide riboside effectively elevates NAD + levels in humans, appears to be well tolerated, and may have potential to improve cardiovascular parameters.
Introduction
Advancing age is the primary risk factor for the development of cardiovascular disease (CVD), which remains the leading cause of morbidity and mortality in industrial and post-industrial societies 1 . The increase in CVD risk with aging is driven largely by adverse changes to arteries, including stiffening of the aorta, and by increases in systolic blood pressure 2 . As such, interventions designed to lower blood pressure and/or improve arterial function hold promise for preventing age-related CVD.
Chronic calorie restriction (CR) prevents the development of arterial dysfunction and increases in blood pressure with aging in rodents 3 , 4 , and lowers arterial stiffness and blood pressure in overweight-obese middle-aged and older adults 5 , 6 . Despite numerous health benefits, adherence to chronic CR remains poor and possibly even unsafe in normal weight older adults 7 – 9 . As such, there is a critical need to establish safe, practical alternatives to regular CR for enhancing cardiovascular function and health with aging in humans 10 .
The recent identification of several key molecular mechanisms responsible for CR-mediated longevity in model organisms has led to an exciting search for “CR-mimetic” interventions to improve cardiovascular and other physiological functions with aging 11 , 12 . In this regard, nicotinamide adenine dinucleotide (NAD + ) has emerged as a critical signaling molecule and essential substrate for sirtuins, a class of enzymes that mediate several of the beneficial effects of CR in model organisms 13 , 14 , including the maintenance of cardiovascular function 15 . Moreover, CR has been shown to increase NAD + levels in pre-clinical models 16 , 17 . The cellular bioavailability of NAD + and related metabolites declines in animals and in humans during normal aging 13 , 18 – 21 and may contribute to physiological aging by reducing sirtuin activity. Although NAD + can be synthesized de novo from the amino acid tryptophan, this process does not occur in all tissues, requiring most cells to rely on a salvage pathway for regenerating NAD + from other intracellular intermediates, which are primarily made available through dietary sources 22 . Vitamin B 3 (niacin: i.e., nicotinic acid and nicotinamide) enters this salvage pathway and acts as a NAD + precursor; however, nicotinic acid is associated with undesirable flushing at therapeutic doses 23 and nicotinamide does not reliably activate (and may even inhibit) sirtuins despite raising concentrations of NAD + 24 – 26 . Therefore, administration of nicotinic acid or nicotinamide is unlikely to be widely adopted for maintaining health and function with aging.
In contrast to these compounds, oral supplementation with either of the NAD + metabolites, nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR), increases levels of NAD + and improves multiple physiological functions in animal models 18 , 27 , 28 . Indeed, we recently demonstrated that supplementation of NMN in the drinking water improved cardiovascular function in old mice 29 . Moreover, CR increases concentrations of NR and NAD + and restores normal circadian gene transcription in the liver, further suggesting that NR may act as a CR mimetic 30 . Thus, NMN and NR are NAD + boosting compounds that hold promise for enhancing cardiovascular health and physiological function with aging 31 , 32 .
Despite these encouraging results from preclinical studies, the tolerability and effectiveness of chronic supplementation with NMN or NR have not been established in humans. Because NR is readily taken up by cells and acts as a direct vitamin precursor for NAD + synthesis 33 , its recent development as a dietary ingredient (NIAGEN ® , ChromaDex Inc., Irvine, CA) has provided the first opportunity to translate the potential benefits of NAD + boosting molecules to people. In this regard, a recent study showed that single doses of NR stimulated blood cellular NAD + metabolism in healthy humans in a dose-dependent manner 26 . However, the tolerability of chronic NR supplementation and its efficacy for increasing NAD + bioavailability have not been established in humans, and we lack even initial insight into the potential of NR for improving cardiovascular and other physiological functions with human aging.
To address these important research gaps, we conducted a small randomized, placebo-controlled, crossover clinical trial of NR supplementation (500 mg, 2×/day) to assess its overall tolerability and efficacy vs. placebo for raising levels of NAD + -related metabolites in healthy middle-aged and older men and women. We also took the opportunity to gain preliminary insight into the effects of chronic NR supplementation for improving cardiovascular and other physiological functions associated with risk of clinical diseases and/or disability with aging. Our results demonstrate that 6 weeks of NR supplementation at this dose is well-tolerated in humans and effectively increases blood cellular NAD + concentrations. Exploratory analyses of the effects of chronic NR supplementation on physiological function in this cohort of healthy middle-aged and older adults suggest that the potential for reducing systolic blood pressure and arterial stiffness may be the most promising hypotheses to investigate in future larger-scale clinical trials, particularly in individuals with elevated baseline blood pressure.
Subject enrollment and baseline characteristics
Information on subject consent, randomization, testing and completion is presented in Fig. 1 . Sixty healthy middle-aged and older men and women between the ages of 55 and 79 years were consented for this study, which was registered on clinicaltrials.gov under the identifier {"type":"clinical-trial","attrs":{"text":"NCT02921659","term_id":"NCT02921659"}} NCT02921659 and conducted between March 2015 and September 2016. The individuals recruited for this study were lean (average BMI = 24 ± 4 kg m −2 ) and healthy, and were representative of the late middle-aged/older adult population within the greater Boulder County Colorado community. Twenty-five participants did not meet inclusion criteria and were excluded without being randomized. Four participants dropped out of the study prior to randomization due to a conflict with time commitment, and one individual was unresponsive to scheduling requests, resulting in a total of 30 subjects remaining for randomization. Of these, 15 subjects were randomized to Group A, which received placebo capsules during the first 6 weeks of the study before crossing over to receive NR capsules for the remaining 6 weeks. The other 15 subjects were randomized to Group B, which received NR capsules first followed by placebo. One subject was withdrawn from Group A due to a change in medication status that no longer met inclusion criteria, and two subjects in Group A elected to drop out of the study due to a complaint of side effects (see below). Two subjects were withdrawn from Group B due to a change in health or medication status that no longer met inclusion criteria, and one subject elected to drop out of Group B due to a non-study-related injury, resulting in a total of 24 subjects who completed the trial. Removal of these six subjects did not influence the overall makeup of the group because the characteristics for the 24 subjects who completed the trial were similar to those for all 30 subjects who were initially randomized (Supplementary Table 8 ). The subjects that completed the study were well matched between groups for age, sex and clinical characteristics, and all baseline values were within normal clinical ranges (Table 1 ).

Study flow diagram
Baseline subject characteristics
| Subject characteristic | Group A | Group B | All subjects combined |
|---|---|---|---|
| Sex (M/F) | 5/7 | 6/6 | 11/13 |
| Age (years) | 64 ± 6 | 66 ± 9 | 65 ± 7 |
| Mass (kg) | 67 ± 16 | 69 ± 14 | 68 ± 15 |
| BMI (kg m ) | 23 ± 4 | 24 ± 3 | 24 ± 4 |
| Total body fat (%) | 27 ± 10 | 29 ± 11 | 28 ± 10 |
| Systolic blood pressure (mmHg) | 122 ± 18 | 120 ± 17 | 121 ± 17 |
| Diastolic blood pressure (mmHg) | 77 ± 11 | 72 ± 9 | 74 ± 10 |
| Fasting glucose (mg dl ) | 89 ± 8 | 87 ± 8 | 88 ± 8 |
| Total cholesterol (mg dl ) | 192 ± 38 | 183 ± 36 | 187 ± 36 |
| HDL cholesterol (mg dl ) | 69 ± 12 | 69 ± 25 | 69 ± 19 |
| LDL cholesterol (mg dl ) | 107 ± 36 | 94 ± 23 | 101 ± 30 |
Includes all subjects who completed the study ( N = 24) by randomization group (A = placebo, NR; B = NR, placebo) and in all subjects combined. Data are mean ± SD.
BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein
Treatment-emergent adverse events
Adherence to the study treatments was excellent, with all subjects consuming greater than 95% of all NR and placebo capsules administered. NR was well tolerated at the dose tested, and no serious adverse events occurred. A total of 14 treatment-emergent adverse events (AEs) were reported by 7 of the 30 participants enrolled in the study, with the other 23 subjects reporting no AEs. All self-reported AEs were mild in severity. The reported symptoms included nausea, flushing, leg cramps and increased bruising during the NR condition, and headache, skin rash, flushing, fainting and drowsiness during the placebo condition (Table 2 ) . Only 2 out of the 30 enrolled subjects (<10%) dropped out of the study due to a complaint of side effects, both occurring while subjects were in the placebo phase (headache and skin rash); no subject dropped out during the NR treatment condition.
Treatment-emergent adverse events (AEs)
| Adverse event (AE) | Placebo No. of events (no. of events/patient) | NR No. of events (no. of events/patient) |
|---|---|---|
| Headache | 4 (1) | 0 (0) |
| Nausea | 0 (0) | 1 (1) |
| Skin rash | 1 (1) | 1 (1) |
| Flushing/Hot flashes | 2 (1) | 1 (1) |
| Fainting | 1 (1) | 0 (0) |
| Drowsiness | 1 (1) | 0 (0) |
| Leg cramps | 0 (0) | 1 (1) |
| Increased bruising | 0 (0) | 1 (1) |
Data represent number ( n ) of times AE was reported. Number of subjects reporting AEs ( n = 7); Number of subjects reporting ≥2 AE ( n = 5)
Obtained from self-report during bi-weekly check-in visits over each phase. Based on N = 30 randomized subjects
Clinical laboratory values were obtained from blood samples collected at the end of each treatment phase in 21 of the 24 subjects who completed the study. Complete blood work could not be obtained from the remaining three subjects due to failed catheterization ( n = 1), administrative error ( n = 1) or study nurse error ( n = 1). No meaningful differences were observed between treatment conditions for hematology (Supplementary Table 1 ), blood chemistry, including markers of renal function and liver enzymes (Supplementary Table 2 ), or blood lipid profiles (Supplementary Table 3 ). Importantly, all clinical laboratory values remained within the normal reference range during both the placebo and NR conditions. Collectively, these results indicate that oral supplementation with NR for 6 weeks at this dose is well-tolerated in healthy middle-aged and older adults.
Efficacy of NR for increasing NAD + and related metabolites
After demonstrating the tolerability of chronic NR supplementation, our primary objective was to determine if NR raises blood cellular NAD + metabolism in humans. Because blood NAD + and several related metabolites of interest have recently been shown to be measurable in circulating peripheral blood mononuclear cells (PBMCs), but undetectable in plasma and urine 26 , we assessed the NAD + metabolome in circulating PBMCs, as previously established 34 .
Oral NR supplementation effectively elevated levels of NAD + in PBMCs by ~60% compared with placebo (mean change = 6.2 pmol per mg protein; one-sided 95% CI (0.074, ∞)). The mean level of NADP + also increased, but did not reach statistical significance (mean change = 1.2 pmol per mg protein; one-sided 95% CI (−2.15, ∞)) (Fig. 2 and Table 3 ). Of note, NR also elevated levels of nicotinic acid adenine dinucleotide (NAAD) nearly fivefold above the placebo condition (mean change = 1.1 pmol per mg protein; 95% CI (0.26, ∞)), confirming a previous report that NAAD is a highly sensitive and reliable biomarker of increased NAD + metabolism and a product of NR utilization in humans 26 . NR also elevated the mean concentration of nicotinamide (NaM), but this was not statistically significant (mean change = 106.5 pmol per mg protein; one-sided 95% CI (−10.03, ∞)). An increase in NaM would suggest an increase in the activity of NAD + -consuming enzymes, which catalyze the breakdown of NAD + into NaM and ADP-Ribose 35 . Though not significant, we also observed an ~1.5-fold increase in NMN (mean change = 0.72 pmol per mg protein; one-sided 95% CI (−0.60, ∞)), which may indicate the possible conversion of NR to NMN by nicotinamide riboside kinase (NRK) enzymes or further metabolism of NaM into NMN by nicotinamide phosphoribosyltransferase (NAMPT) 35 . Consistent with the only other report of NR ingestion in humans 26 , we were unable to detect NR concentrations in PBMCs during either treatment condition, despite using optimized recovery methods. The magnitude by which NAD + increased in response to NR supplementation was negatively associated with blood cellular NAD + concentration during the placebo condition ( R = −0.49, R 2 = 0.25), suggesting a greater response in individuals with naturally low blood cellular NAD + levels.

NAD + metabolome. NAD + and related metabolite concentrations in peripheral blood mononuclear cells increased after oral placebo vs. NR supplementation normalized to total protein content. Data are mean ± SD. * indicates unadjusted P < 0.05 by one-tailed paired t- test. N = 21 (Group A = 11; Group B = 10)
NAD + metabolites
| Metabolite | Median | Range | -value | ||
|---|---|---|---|---|---|
| Placebo | NR | Placebo | NR | ||
| NAAD | 0.0 | 0.0 | 0.0−2.3 | 0.0−8.7 | 0.018* |
| NAD | 7.7 | 12.2 | 0.0−27.4 | 4.7−67.8 | 0.048* |
| NADP | 6.1 | 6.3 | 3.3−17.9 | 2.7−42.7 | 0.267 |
| NaM | 257.6 | 278.6 | 109−411 | 171−1357 | 0.065 |
| NMN | 0.0 | 0.0 | 0.0−5.5 | 0.0−11.9 | 0.179 |
| ATP | 1592 | 2205 | 363−3446 | 763−5459 | 0.032 |
All values expressed as pmol per mg protein. * represents unadjusted P < 0.05; ATP represents secondary outcome assessed at Bonferroni-adjusted P < 0.006
In addition to boosting NAD + -specific metabolites in PBMCs, we also observed increases in the mean concentration of other metabolites involved in the regulation of energy production and metabolism, including adenosine and adenosine triphosphate (ATP; mean change = 699 pmol per mg protein; one-sided 95% CI (84, ∞); Fig. 2 and Table 3 ); however, analysis of this metabolite was considered a secondary outcome and the increase did not attain statistical significance after correction for multiple comparisons. NR supplementation also tended to raise levels of adenosine diphosphate (ADP) and adenosine monophosphate (AMP), though increases in these metabolites did not reach statistical significance (Supplementary Table 4 ). Collectively, these findings indicate that chronic NR supplementation effectively stimulates NAD + metabolism in healthy middle-aged and older men and women.
Effect of NR on indicators of cardiovascular health
Supplementation with NR tended to lower mean systolic (SBP; mean change = −3.9 mmHg; one-sided 95% CI (−∞, −0.058)) and diastolic (DBP; mean change = −2.0 mmHg; one-sided 95% CI (−∞, −0.26)) blood pressure (BP) in all subjects as a group (Fig. 3a–c ); however, these comparisons were not statistically significant after correction for multiple comparisons. Because the risk of cardiovascular events is greatly increased in individuals with above-normal baseline BP 36 , we performed a follow-up analysis to compare the effect of NR on BP in the participants with BP in the normal range (SBP/DBP < 120/80 mmHg; N = 11) vs. those with BP in the elevated/stage I hypertension range (SBP, 120–139 mmHg; DBP, 80–89 mmHg; N = 13) based on recently updated guidelines 37 . Of particular note, mean SBP was 9 mmHg lower after NR vs. placebo in individuals with elevated/stage I hypertension, whereas no change was observed in subjects with initial SBP in the normal range (Fig. 3d ). Because this post-hoc subgroup analysis was exploratory, no statistical inferences can be made. The median values and ranges for all blood pressure variables are provided in Supplementary Table 6 .

Blood pressure. Effect of 6 weeks of oral placebo vs. NR supplementation on a systolic (SBP) and b diastolic (DBP) blood pressure, and c pulse pressure (PP) in healthy middle-aged and older adults as a whole N = 24 (Group A = 12; Group B = 12), and overall change from placebo in blood pressure parameters ( d−f ) in subjects with normal ( N = 11) vs. above normal ( N = 13) baseline BP. Data are mean ± SD. P- values reported in individual bars based on a one-tailed paired t -test (panels a − c only) and an adjusted alpha level set at 0.006
We also observed a trend towards a reduction in the mean carotid-femoral pulse wave velocity (PWV) with NR supplementation, the clinical “gold standard” measure of the stiffness of the aorta 38 , and a strong independent risk factor for incident cardiovascular events with aging and age-related diseases (Fig. 4a ; mean change = −41.5 m s −1 ; one-sided 95% CI (−∞, −4.8)). However, this reduction was not statistically significant after correction for multiple comparisons. Similar to our exploratory analysis of BP, NR supplementation tended to lower aortic stiffness (carotid-femoral PWV) more in individuals with higher baseline BP (Fig. 4b ), although no statistical inferences were made for this post-hoc comparison. No effect of NR was observed on ultrasound-determined carotid artery compliance (Fig. 4c ) or brachial artery flow-mediated dilation, a measure of vascular endothelial function (Fig. 4d ).

Arterial function. Effect of 6 weeks of oral placebo vs. NR supplementation on a aortic pulse wave velocity (PWV) as a whole ( N = 24; 12 per group), b subgroups of individuals with normal ( N = 11) vs. above-normal ( N = 13) baseline BP); c carotid artery compliance (CC) and d brachial artery flow-mediated dilation (FMD) in the overall groups ( N = 24; 12 per group). Data are mean ± SD. P -values reported in individual bars based on one-tailed paired t -test (panels a , c , and d only) and an adjusted alpha level set at 0.006
Effect of NR on other domains of physiological function
To gain exploratory insight into potential benefits of NR supplementation on other domains of physiological function in healthy middle-aged and older adults, we assessed a wide variety of outcomes indicative of metabolic function, motor function, and exercise capacity/performance. Total energy intake and expenditure, oxidative fuel source (carbohydrate vs. fat), and physical activity patterns were not affected by NR (Supplementary Table 7 ). Likewise, we observed no difference in body mass, body mass index (BMI) or percent body fat compared with the placebo arm (Supplementary Table 7 ) and no differences were observed in measures of glucose or insulin regulation (Supplementary Table 7 ). Finally, there was no effect of the intervention on overall motor function (Supplementary Figure 2 ), maximal exercise capacity, as assessed by VO 2 max and treadmill time to exhaustion (Supplementary Figure 1A, B ), or on markers of submaximal exercise performance (Supplementary Figure 1C−F ).
The primary finding of this study is that chronic oral supplementation with 1000 mg per day of NR is a well-tolerated and effective strategy for stimulating NAD + metabolism in healthy middle-aged and older humans. Additionally, our exploratory analyses of the effects of NR supplementation on physiological function suggest that the ability of NR to reduce SBP and aortic stiffness, two clinically important risk indicators of cardiovascular function and health, are among the most promising hypotheses to test in a future larger-scale clinical trial, particularly in individuals with above-normal baseline SBP.
In this small initial intervention trial, NR was well tolerated and elicited no serious adverse effects. Additionally, we found that NR stimulated NAD + metabolism without any difference in treatment-emergent AEs compared with placebo, supporting previous suggestions that NR may be a more suitable NAD + precursor than niacin (i.e., nicotinic acid and nicotinamide), which` also is capable of entering the NAD + salvage pathway, but is associated with a painful flushing sensation at therapeutic doses 22 , 23 . The flushing response induced by niacin is specifically caused by the binding of nicotinic acid to the Gpr109A receptor on epithelial cells 39 , 40 , an action that is not expected to occur with NR, thereby minimizing the risk of this side effect at equivalent doses 22 . Although three of the subjects in our study did report flushing, two of these subjects were taking the placebo capsules, suggesting that NR itself did not appear to be associated with flushing. Despite these promising findings, we wish to emphasize that the size of the cohort in the present study is insufficient to establish the broader safety profile of NR at this dose. Larger, more definitive clinical trials, similar to those conducted with niacin, will be necessary to confirm this preliminary evaluation of tolerability and to more definitively assess its overall safety. However, indirect evidence for the potential safety of NR supplementation is suggested by the widespread use of niacin over past 60 years for the treatment of high cholesterol, with limited side effects other than flushing 41 .
Niacin serves as important dietary precursor to NAD + and helps sustain cellular function and protect against pellagra, a condition characterized by dark pigmented skin, dermatitis, diarrhea, and dementia. Like niacin, NR has been detected in cow’s milk 42 , 43 and may theoretically act as another vitamin precursor form of NAD + . However, the amount of NR naturally consumed through the diet is likely much smaller than the dose tested in the present study. The primary natural sources of vitamin B 3 come from NAD + , NADPH and NADH, which are more abundant in food sources and are broken down into salvageable precursors, including niacin and NR. To protect against pellagra, the recommended daily allowances of niacin for adult men and women have been set at 16 and 14 mg per day, respectively 22 , whereas higher doses of niacin have beneficial effects on lipid profiles of individuals at risk for cardiovascular events 23 . It is plausible that the daily requirement for NAD + precursors may increase with advancing age due to decreasing NAD + bioavailability, although no such recommendation presently exits.
An important goal of the present study was to identify clinically relevant physiological outcomes for future larger-scale (phase II) clinical trials of NR supplementation. The most promising result of these exploratory analyses was a trend towards an improvement in selective indicators of cardiovascular function. Compared with placebo, NR tended to lower SBP and aortic stiffness, two major independent risk factors for incident cardiovascular events and disease with advancing age 2 , 36 , in the overall group. A follow-up analysis suggested that this trend was most pronounced in individuals with baseline BP between 120 and 139 mmHg, a subgroup currently classified clinically as having either “elevated” SBP (120–129 mmHg) or stage 1 systolic hypertension (130–139 mmHg). The mean decrease in SBP after NR treatment in this subgroup approached 10 mmHg—a magnitude of change associated with a 25% decrease in incident CV events in a recent major anti-hypertensive drug trial in older adults 44 . If this magnitude of SBP reduction with NR supplementation is confirmed in a larger clinical trial, such an effect could have broad biomedical implications. SBP in this range (120−139 mmHg) is observed in ~50% of all middle-aged and older adults in the U.S. 45 Moreover, SBP < 140 mmHg is responsible for at least one-third of all BP-attributable deaths 46 and is associated with increased risk of heart disease, stroke, cognitive impairment/dementia and chronic kidney disease, among other disorders of aging 47 . Importantly, for individuals with SBP of 120–139 mmHg, lifestyle modifications, such as healthy diet and regular exercise are recommended before prescribing anti-hypertensive medications 37 . Given the low adherence to healthy lifestyle practices in middle-aged and older adults 7 , a natural, CR-mimicking, dietary supplement like NR with potential BP-lowering effects might represent a complementary approach for preserving cardiovascular health with aging.
We also observed a trend towards a reduction in carotid-femoral PWV after NR supplementation in the present study, suggesting that this measure of aortic stiffness may represent another promising cardiovascular outcome of interest for a larger future clinical trial. As with the effects on SBP, individuals with higher baseline BP appeared to demonstrate the largest mean changes in carotid-femoral PWV in response to NR treatment. Carotid-femoral PWV is not only independently predictive of incident CVD in older adults, but has recently been linked to numerous other age-associated diseases and disorders including mild cognitive impairment, chronic kidney disease, and frailty 48 – 51 . Thus, healthy lifestyle-mimicking dietary supplements with the potential to reduce age-related stiffening of the aorta would be of significant clinical interest. It should be noted that any NR-associated changes in BP may have contributed to corresponding changes in carotid-femoral PWV (and vice versa), so the changes in SBP and arterial stiffness are interrelated 52 – 54 .
Little is known about the underlying mechanisms by which NAD + precursors may reduce BP and aortic stiffness in humans. NAD + is an obligate substrate for the deacetylase sirtuin 1 (SIRT-1), which is implicated in the maintenance of healthy vascular function 3 , 15 , 55 . In this regard, treatment with the proposed pharmacological SIRT-1 activator SRT1720 protects against the development of aortic stiffness and hypertension in Klotho-deficient mice (a model of accelerated aging), by lowering vascular oxidative stress 56 . Likewise, we have demonstrated that supplementation with NMN reverses aortic stiffening in old mice to youthful levels by increasing aortic SIRT-1 activation and reversing age-related increases in aortic oxidative stress, collagen deposition and elastin fragmentation 29 . Based on these preclinical studies, it is possible that NR may similarly affect BP and aortic stiffness in humans through a mechanism involving SIRT-1 activation; however, future mechanistic studies are needed to test this and related hypotheses. Such studies will be technically challenging in humans, and it will be important to try to separate the effects of SIRT-1 activation from the likely pleotropic effects of boosting the NAD + metabolome.
In addition to cardiovascular parameters, we also assessed the effect of NR on other domains of physiological function. Our findings suggest that a relatively short (6-week) intervention with NR did not change total energy expenditure or energy expenditure from fat oxidation (based on assessment of RER) at rest. We also did not observe any improvement in blood glucose control or insulin sensitivity. In both cases, it is important to note that this study was conducted in lean, healthy middle-aged and older adults without baseline metabolic dysfunction. These observations are in agreement with previous findings that NR modulates resting metabolic rate only in mice exposed to a high-fat diet 57 , and does not influence blood glucose regulation in animals fed normal chow diets 58 . Based on these preclinical observations, it is possible that chronic NR supplementation might improve metabolic function in groups who consume unhealthy diets and/or have metabolic disorders such as obesity, diabetes, and/or the metabolic syndrome.
Our results also suggest that NR does not obviously improve aerobic exercise capacity or motor function in healthy middle-aged and older men and women with good baseline physical status. Although Frederick et al. 28 recently identified a role for NAD + in rescuing neuromuscular function and exercise capacity in mice, these studies were conducted using a genetically induced model of impaired NAD + bioavailability that may represent more severe NAD + depletion than that which occurs with healthy aging. Future studies should explore the role of NR supplementation on aerobic exercise capacity and motor performance in groups with impaired mobility such as frail older adults and in individuals with chronic diseases associated with reduced cardiorespiratory fitness and exercise intolerance.
In summary, the results of the present study provide initial evidence that chronic NR supplementation is well-tolerated in healthy middle-aged and older adults, and extend recent findings that acute supplementation with NR is effective for stimulating NAD + metabolism in humans. Furthermore, we provide the first insight into the effects of NR supplementation on physiological function in humans, and identify SBP and aortic stiffness as promising cardiovascular outcomes to be assessed in larger-scale clinical trials. Future work should also compare the relative increases in NAD + and the physiological benefits of NR with conventional CR in order to further evaluate the use of this dietary supplement as a true “CR-mimetic” compound.
Finally, we wish to emphasize certain limitations of this initial trial on chronic NR supplementation in humans. Because the physiological outcomes in this study were designed to be exploratory in nature, the associated statistical inferences for those variables were based on one-sided hypothesis testing and the alpha level was set at a conservative P < 0.006 to account for multiple testing. More targeted studies (e.g., phase-II clinical trials) with fewer outcomes based on two-sided statistical inference are needed to confirm the effects of NR supplementation on SBP and carotid-femoral PWV (aortic stiffness) before moving towards larger-scale (phase-III) clinical trials and any recommendation of NR supplementation for improving these cardiovascular health indicators. It is also important to note that although NR is presently available as a dietary supplement under the trade name NIAGEN ® (ChromaDex, Inc.), the dose tested in the present study exceeds the label-recommended dose and should be considered investigational until further work can be performed to confirm the safety and efficacy of higher doses for use by the general population. Lastly, the present study assessed the influence of chronic NR supplementation on healthy middle-aged and older adults, which may have reduced the likelihood of observing greater or, in the case of several outcomes assessed, any improvements in physiological function. Thus, future investigations should include studies on groups with cardio-metabolic diseases, motor deficits, impaired NAD + metabolism, and/or other disorders to determine the efficacy of NR supplementation for enhancing health status in populations with impaired baseline physiological function.
Ethical approval, informed consent, and study location
All procedures were approved by the University of Colorado Boulder Institutional Review Board. The nature, benefits, and risks of the study were explained to all subjects, and their written informed consent was obtained prior to participation. All measurements were performed at the University of Colorado Boulder Clinical & Translational Research Center (CTRC) and in the Integrative Physiology of Aging Laboratory. The study was registered on ClinicalTrials.gov under the identifier {"type":"clinical-trial","attrs":{"text":"NCT02921659","term_id":"NCT02921659"}} NCT02921659 .
Study participants
Middle-aged and older men and postmenopausal women aged 55−79 years were recruited from Boulder Colorado and surrounding communities. All subjects were free of clinical diseases, including peripheral artery disease (ankle-brachial index >0.90) and overt CVD as assessed by a graded exercise test, baseline blood panel, medical history, and physical examination by a physician. All subjects demonstrated age-related impairments in vascular endothelial function (defined as a flow-mediated dilation value <6%) and were excluded if they exhibited abnormal blood chemistries for renal or liver function (defined as 1 standard deviation outside of the normal range), had alcohol dependence, uncontrolled thyroid disease, severe obesity (body mass index >40 kg m −2 ), or were not weight stable for at least 3 months prior to enrolling in the study (defined as >2 kg change in body mass). Body mass, BMI, and waist and hip circumferences were measured by anthropometry, and total body fat percentage was measured using dual-energy x-ray absorptiometry (Lunar/Prodigy, GE). Fasting glucose and total, LDL, and HDL cholesterol levels were measured using standardized assays at the University of Colorado Boulder CTRC Core Laboratory at baseline and after each intervention phase of the study.
Study design, randomization, and intervention
The study design consisted of a 2 × 6-week randomized, double-blind, placebo-controlled crossover clinical trial. Subjects ingested nicotinamide riboside chloride (NIAGEN ® ; 500 mg, twice per day; ChromaDex, Inc.) and placebo capsules for 6 weeks each in a randomly determined order. Subjects were randomized after providing informed consent and meeting all inclusion criteria. Randomization was performed by a member of the study team not involved in the assessment of outcomes. The study participants and members of the study team involved in the collection and analysis of outcomes were blinded to the treatment condition. Capsules were consumed with meals in the morning and evening. Subjects refrained from taking any over-the-counter medications for 48 h and prescription medications for 24 h prior to all experimental testing. All assessments were performed after a 12 h overnight fast with the exception of motor function tests, which were performed 2 h after a light meal or snack in order to ensure that subjects had enough energy to complete the testing battery. Subjects refrained from consuming alcohol or engaging in vigorous exercise for 24 h and refrained from taking study pills for at least 12 h prior to all testing sessions.
Evaluation of safety and tolerability
Adherence to the intervention was assessed by pill count. Subjects reported to the laboratory every 2 weeks to receive a new bottle of capsules and to discuss any issues with tolerability or treatment-emergent AEs with a member of the research team who was not involved in data collection or analysis in order to ensure blinding of the investigators. Standard clinical markers of hematology, liver and kidney function and blood lipids were analyzed using standardized clinical assays at Boulder Community Hospital and any abnormal blood results were reviewed by the study physician.
Isolation of peripheral blood mononuclear cells
PBMCs were isolated from 35 ml of whole blood collected into EDTA-coated Vacutainer™ tubes. The blood was then centrifuged at 400 × g for 20 min and the majority of the plasma layer (~60%) was removed to increase the efficiency of the downstream PBMC isolation. The remaining sample was slowly added to a new 50 ml conical tube containing 10 ml of Histopaque 1.077 (Greiner Bio-One) and the mononuclear cell layer was isolated by density-dependent centrifugation at 400 × g for 20 min, washed and then frozen in 2 ml of PBS at −80°C.
NAAD, NAD + , NADP + , NaM, NmN, mono-, di- and triphosphate nucleotides and nucleosides were obtained from Sigma Aldrich (St. Louis, MO). Adenosine (ribose-13C5) and adenosine triphosphate (ribose-d4) were obtained from Cambridge Isotope Laboratories (Tewksbury, MA). NaM (13C6) was obtained from Cerilliant (Round Rock, TX). Nicotinamide riboside and doubly labeled nicotinamide riboside (13C1, H 2 -1) were obtained from ChromaDex Inc. (Irvine, CA). All HPLC solvents and extraction solvents were HPLC grade or better.
Preparation of calibration standards
Individual stock standards were prepared by dissolving 10 mg ml − 1 in 1:1 methanol:water and then combining to obtain a stock mixture. The NAD + metabolite combined stock was prepared at 100 μg ml −1 of each compound and the nucleoside/nucleotide combined stock was prepared at 400 μg ml −1 of each compound; both were frozen at −20 °C until use. The internal standard solution was prepared at 250 μg/ml adenosine-13C5, adenosine triphosphate-d4, doubly labeled nicotinamide riboside and 2.5 μg ml −1 of NaM-13C6 in 1:1 methanol:water. Immediately before analysis of each sample batch, the individual calibration curve standards were prepared by combining the NAD + and nucleoside/nucleotide stocks at a ratio of 1:1 and then diluting them in 1:1 methanol:water to the required concentrations. Concentrations ranged from 0.025 to 25 μg ml −1 for the NAD + metabolites and from 0.1 to 100 μg ml −1 for nucleosides/nucleotides. Internal standard concentrations in all calibration levels and samples were 50 μg ml −1 for adenosine-13C5, adenosine triphosphate-d4 and doubly labeled nicotinamide riboside, and 0.5 μg ml − 1 for NaM-13C6.
Extraction of NAD + metabolites and nucleosides/nucleotides
Frozen PBMCs (5×10E6 cells total) were thawed on ice. 500 μl of ice cold 70:30 methanol:water was added along with 20 μl of internal standard and samples vortexed for 10 s. The resulting extract was centrifuged at 8000 × g for 5 min at 4 °C. The resulting supernatant was transferred to a new centrifuge tube and stored on ice. To the remaining pellet, 500 μl of ice cold methanol was added and the sample vortexed for 10 s to resuspend the pellet. The sample was then centrifuged at 8000 × g for 5 min at 4 °C. The entire supernatant was removed and combined with the 70% methanol supernatant. The resulting pellet was reserved and frozen at −70 °C for protein concentration analysis using the Bradford assay. The combined supernatants were centrifuged at 18,000 × g for 15 min and the resulting supernatant was transferred to a new tube and dried in a vacuum centrifuge at 55 °C. The dried samples were reconstituted in 100 μl of 1:1 methanol:water and centrifuged at 18,000 × g for 10 min at 4 °C. The supernatant was then transferred to a reduced surface activity autosampler vial for analysis.
HPLC separation of NAD + metabolites and nucleosides/nucleotides was performed using a method described by Evans et al. 59 with minor modifications. Separation of NAD + metabolites and nucleosides/nucleotides was performed on a 1200 series HPLC from Agilent (Santa Clara, CA) using a 100 × 2 mm 5 μm Luna NH2 column from Phenomenex (Torrance, CA) operated in HILIC mode. Buffer A consisted of 100% acetonitrile and buffer B consisted of 95:5 water with 20 m m ammonium acetate adjusted to pH 9.6 with 20 m m ammonium hydroxide. Ten microliters of the extracted sample was analyzed using the following gradient at a flow rate of 0.6 ml per min: linear gradient from 5 to 100% B over 6 min, hold at 100% B from 6 to 9.5 min, then 100−5% B from 9.5 to 10.5 min, followed by re-equilibration at 5% B from 10.5 to 14 min. The column temperature was held at 15 °C for the entire gradient. Mass spectrometric analysis was performed on an Agilent 6410 triple quadrupole mass spectrometer in positive ionization mode. The drying gas was 300 °C at a flow rate of 12 ml per min. The nebulizer pressure was 30 psi. The capillary voltage was 4000 V. Data for NAD + metabolites and nucleosides/nucleotides were acquired in MRM mode using experimentally optimized conditions obtained by flow injection analysis of authentic standards (Supplementary Table 5 ). Calibration standards were analyzed over a range of concentrations from 0.25 to 250 ng on column for the NAD + metabolites and from 1 to 1000 ng on column for nucleosides/nucleotides. Calibration curves for each NAD + metabolite and nucleoside/nucleotide were constructed using Agilent Masshunter Quantitative Analysis software. Results for PBMCs were quantitated using the calibration curves to obtain the on-column concentration, followed by normalization of the results using the protein concentration of the pellet reserved from the PBMC extraction.
Assessments of cardiovascular function
Resting blood pressure was measured in the seated position after at least 10 min of quiet rest using a semi-automated blood pressure device (Dynamap™ XL, Johnson & Johnson, Arlington, TX, USA). Measurements were made multiple times from the non-dominant arm, with 2 min of quiet rest between recordings. Repeat measurements were made until three blood pressure values were obtained that were within 5 mmHg of one another. These values were then averaged to determine resting systolic and diastolic blood pressure and pulse pressure. Baseline blood pressure values were obtained using the above-described protocol on two separate testing days prior to the initiation of the first intervention arm and were averaged to determine baseline blood pressure status (i.e., normal vs. above normal) for subsequent analyses.
Aortic stiffness was measured using carotid-to-femoral PWV, the gold-standard assessment of elastic artery stiffness in humans 38 . Pressure waveforms were recorded simultaneously from the carotid and femoral arteries using applanation tonometry (Millar Inc., Houston, Texas) as previously described by our laboratory 60 – 62 . The transit time of the aortic pulse wave was determined by measuring the time-delay between the foot of the carotid and femoral pressure waves using LabChart analysis software. PWV was calculated by dividing the distance between the two measurement sites by the aortic transit time.
Carotid artery compliance was determined by the change in diameter of the right common carotid artery (assessed using high resolution ultrasonography, PowerVision 6000, Toshiba) relative to the change in carotid blood pressure (assessed using applanation tonometry, Millar Inc., Houston, TX) across the cardiac cycle. Carotid pressure was normalized to brachial artery pressure obtained using an automated blood pressure cuff (Dynamap™ XL, Johnson & Johnson, Arlington, TX, USA). Compliance was calculated as CC = π × DD 2 × (ΔD DD −1 )/(2 × PP), where DD is diastolic diameter, ΔD is the change in diameter and PP is the arterial pulse pressure, as has been described previously 62 – 65 .
Endothelium dependent dilation was measured as brachial artery flow-mediated dilation (FMD) to reactive hyperemia, using high-resolution ultrasonography (PowerVision 6000, Toshiba) as previously described 66 – 68 . FMD was expressed as the percentage change (%Δ) from baseline diameter.
Assessments of metabolic function
Three-day dietary records were collected at baseline and during the last week of each intervention phase to ensure stability of caloric intake. Results were analyzed by a registered dietician using the Nutrition Data System for Research (University of Minnesota) as previously described by our laboratory 67 , 69 .
Resting metabolic rate was measured by indirect calorimetry (ParvoMedics TrueOne 2400) as described previously by our laboratory 70 , 71 . Subjects rested in a supine position for 45–60 min with a ventilated hood placed over their head to collect concentrations of expired oxygen (O 2 ) and carbon dioxide (CO 2 ). Metabolic rate and respiratory exchange ratio (RER) were calculated in 1-min segments and averaged from at least 30 min of steady data.
Insulin sensitivity was assessed by measuring insulin-stimulated whole-body glucose uptake using a modified frequently sampled intravenous glucose tolerance test and the Minimal Model Method of analysis as described in detail elsewhere 72 . Insulin resistance and beta cell sensitivity were assessed using the homeostasis model assessment (HOMA) method as previously described 73 .
Assessments of exercise capacity and physical function
Cardiorespiratory fitness was determined from a graded treadmill exercise test to volitional exhaustion using a modified Balke protocol as previously described 74 . Oxygen consumption (VO 2 ) and RER were measured using open-circuit spirometry with an online, computer-assisted analysis system. Heart rate and ratings of perceived exertion (RPE) were also measured throughout the test.
Walking endurance was assessed by measuring the distance covered during a 6-min walking task on a 50-foot (out-and-back) indoor course as previously described 75 .
Muscle strength and rate of torque development were quantified by measuring the peak force produced during a maximal voluntary contraction, and rate of torque development was measured using the maximal rate of developing torque during a rapid, forceful contraction of the knee flexor and extensor muscles as previously described by our laboratory 76 . Handgrip strength was measured using a standard handgrip dynamometer.
Leg fatigability was assessed using performance until failure during a single-leg heel-rise task. Subjects were asked to perform one complete plantar flexion contraction every 2 s until failure, and the test was terminated when the subject voluntarily stopped due to discomfort or inability to achieve at least 50% of maximal plantar flexion without using upper extremities for more than balance 77 . The chronic attribute of fatigue was also assessed using a Fatigue Questionnaire and Fatigue Severity Scale 78 .
Dynamic balance was assessed using a rapid step test. Maximal step length was measured in each direction (forward, backward, left, right), and targets were placed on the ground at 80% of the subject’s maximum with lines of colored tape, as described previously 79 . Performance was quantified as the time taken and number of errors committed on average during three rounds of the rapid step balance test. Each round consisted of 18 commands instructing subjects to step with a random foot to a random direction (i.e. left front). An error was defined as failure to completely step beyond the target, loss of balance, failure to return to the initial starting position, taking multiple steps to completely reach a target, or stepping with the incorrect leg or to the wrong target.
Mobility was assessed as the time to complete a 4-m walk task (performed in duplicate at the subjects' preferred walking speed) and the five-repeated sit-to-stand test (performed in triplicate), as previously described 75 , 78 , 80 . The test involves rising from a seated position in a standard-height chair five times, as quickly as possible, without using their arms for momentum or support.
Manual dexterity was assessed as the time to complete a 9-hole pegboard test as previously described 76 . Subjects collected smooth, rounded pegs from a dish and placed them into a pegboard, then returned them to the dish as quickly as possible. Two trials were completed with each hand.
Statistical analyses
The sample size for this study was sufficient to detect at least a 50% increase in NAD + concentration following NR supplementation vs. placebo (effect size = 0.7; mean of difference = 7; 1− β = 0.8; α = 0.05) as well as a clinically relevant improvement in the cardiovascular parameter with the lowest effect size (FMD; mean difference = >1%; effect size = 0.86). Estimate of effect size for NAD + was determined from preliminary data of NAD + metabolite concentrations in PBMCs collected from human subjects. Effect size for FMD was determined from our laboratory’s previous crossover interventions demonstrating improvements in vascular function 67 . The required sample size was determined to be 19 subjects. Assuming a 20% dropout (4 subjects) and 40% exclusion due to screen failures (consistent with other intervention studies in our laboratory using dietary supplements 81 , 82 ), a total of 60 participants were consented for this study. Significance was set at α = 0.006 for all secondary outcomes to adjust for multiple testing of NR vs. placebo (paired t- tests) on each of the following nine pre-specified hypotheses: (1) NAD + metabolites, (2) cardiovascular parameters, (3) hematology, (4) metabolic panel, (5) lipid profiles, (6) energy balance, (7) glycemic control, (8) motor function and (9) exercise performance. Because many of the measures within each hypothesis are correlated with one another (e.g., cardiovascular measures, Supplemental Table 9 ), each group of measures listed above was treated as one outcome when adjusting for multiple comparisons. With the exception of our primary outcome variables (NAD + metabolites: NAD + , NAAD, NADP, NaM, NMN), in which inferences were based on an unadjusted alpha level set at 0.05, all inferences of significance are based on the Bonferroni-adjusted alpha level ( α = 0.006).
The intent of this study was to translate promising preclinical evidence for the efficacy of chronic supplementation with NAD + boosting compounds to humans. Therefore, each outcome was tested under a directional hypothesis that was determined a priori, based upon previous studies reported in the literature. Accordingly, one-tailed hypothesis tests were used to compare the proposed unidirectional effects of NR supplementation vs. placebo on these outcomes. This method has been recommended elsewhere for Phase I and II placebo-controlled clinical trials in which the goal is to gain early insight into the potential efficacy of a compound 83 .
Prior to analysis, all continuous outcome variables were assessed for normality using the Shapiro−Wilk test and by examining individual frequency histograms for each outcome. If a variable was non-normally distributed, it was log-transformed prior to analysis. If log-transformation did not normalize the data, treatment condition was analyzed using the non-parametric Wilcoxon signed rank test. For each variable, any subject with a missing value during either phase was excluded from that analysis. Based on the interpretation of the primary data, post-hoc analyses were performed to compare the change in blood pressure and aortic stiffness between subjects who exhibited normal vs. above normal baseline blood pressure using an un-paired two-tailed t- test. We also explored the relation between baseline NAD + concentrations and the overall increase in NAD + using a Pearson correlation. A formal washout period was not included in the study design; however, given the crossover design, we tested for presence of a carryover effect for each of the outcomes under study using linear regression modeled with an indicator for treatment order (no carryover effects were observed between conditions). All statistical analyses were performed using the R statistical computing platform (version 3.2.2) and GraphPad Prism 7 software.
Data availability
Electronic supplementary material
Acknowledgements
We wish to acknowledge Natalie de Picciotto and Laura Stauber for their assistance with subject recruitment and scheduling. We would also like to acknowledge Joe Gomez, Roger Powell, Kevin Quinn, and Samantha Bokatzian for their technical assistance with the development of the NAD + metabolite assays. This work was supported by NIH grants AG000279 and TR001082 and was completed while C.R.M. was a Glenn/AFAR Postdoctoral Fellow. Study pills, NR standards for metabolite analyses and partial funding support were provided by ChromaDex, Inc.
Author contributions
C.R.M. and D.R.S. conceived and developed the overall study design and drafted the manuscript. C.R.M., B.A.D. and M.R.M. collected and analyzed all of the data. M.L.A. and N.R. developed and ran the assay to measure NAD + metabolites. M.B.M. assisted with statistical analysis. M.C. provided medical oversight of the study subjects, evaluated inclusion/exclusion criteria, and reviewed adverse events. All authors edited and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Supplementary Information accompanies this paper at 10.1038/s41467-018-03421-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Get 7 Days Free
ChromaDex Receives Exclusive U.S. FDA Orphan Drug Designation (ODD) and Rare Pediatric (RPD) Disease Designation for Nicotinamide Riboside Chloride (NRC) for the Treatment of Ataxia Telangiectasia (AT)
ChromaDex plans to conduct additional studies on NRC in anticipation of filing for an Investigational New Drug Application (IND) for the treatment of AT
ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company’s product candidate for the treatment of Ataxia Telangiectasia (AT). Plans are underway to file an Investigational New Drug (IND) application with the U.S. FDA in anticipation of conducting human clinical trials, which will be guided by Dr. Vilhelm (Will) Bohr, Prof., University of Copenhagen and Scientific Advisor to ChromaDex.
“ Over 30 million people in the U.S. are impacted by more than 7,000 rare diseases, many of which are life-threatening and lack effective treatments,” said Rob Fried, CEO of ChromaDex. “We believe NRC has potential as a treatment for AT.”
“AT is a condition where children suffer from the adverse effects of premature aging and face a very limited life expectancy,” said Dr. Andrew Shao, ChromaDex Senior Vice President of Global Scientific & Regulatory Affairs. “We are excited to continue supporting the AT community and eagerly anticipate the results from future research.”
About Ataxia Telangiectasia (AT)
Ataxia Telangiectasia (AT) is a rare, progressive disease that typically presents in early childhood and is characterized by neurological and immunological symptoms. Those with AT often exhibit an unsteady gait (ataxia), impaired coordination of eye movements (oculomotor apraxia), and involuntary movements (choreoathetosis). AT leads to cerebellar degeneration and many affected children become wheelchair-dependent. Currently, there is no cure or FDA-approved treatment to slow the progression of AT, with the average life expectancy being around 25 years for those diagnosed in childhood.
There are many types of ataxia. Another form of ataxia is Friedreich’s ataxia (FA), which is being addressed by Biogen and Larimar Therapeutics. FA impacts 1 in 50,000 people in the U.S. ( NIH ). Ataxia Telangiectasia (AT) impacts roughly 1 in 40,000 people in the U.S. (Riboldi et al., 2023; Tieve et al., 2015).
Clinical Research on NRC and AT
To date, NRC has been investigated in two third-party funded, peer-reviewed published clinical trials for the treatment of AT. The first study published in Movement Disorders demonstrated that supplementation with NRC improved AT scores and increased immunoglobulins, or antibodies, in the immune-compromised patients, with AT score improvements reversing once supplementation concluded (Veenhius et al., 2021).
The second phase II two-year long study, also published in Movement Disorders , demonstrated that long-term NRC supplementation increased whole blood NAD + levels up to fourfold, and improved neuromotor coordination and eye movements in 90% of participants while maintaining biomarkers of stable liver and kidney function, as compared to historical disease progression (Presterud et al., 2023). Both studies reported no serious adverse events, with NRC being generally well-tolerated.
Not associated with ChromaDex’s future NRC IND filing, there are two additional investigator-initiated ongoing registered clinical trials that will examine NR supplementation in AT patients. The first is a continuation of Presterud et al. 2023 and will track AT patients over the course of 8-10 years. Recently registered, the second will be a single-arm open-label clinical trial scheduled to commence this year in Australia.
Significance of Orphan Drug Designation (OOD) and Rare Pediatric Disease Designation (RPD)
According to the FDA , there are too few treatments for rare diseases because of high research and development costs, which companies often cannot recoup as a result of small patient populations. To incentivize companies to invest in bringing treatments to market for rare diseases, in 1983, Congress passed the Orphan Drug Act, which makes Orphan Drug Designation (ODD) candidates eligible for tax credits, waives their user fees, and may provide a period of exclusivity should the orphan drug be subsequently approved by FDA.
Related to ODD, the FDA’s Rare Pediatric Disease (RPD) designation , further incentivizes companies to invest in rare childhood diseases by providing a voucher program to applicants approved by September 30th, 2024. Through this program, companies with RPD designation that ultimately obtain successful drug approval for a rare pediatric disease are provided a voucher, which can be used to expedite the FDA review of another drug candidate or sold to other companies.
Future Clinical Trials on NRC and AT
With both designations granted, ChromaDex plans to file an Investigational New Drug (IND) application for future human clinical trials for the use of NRC in the treatment of AT. As a leading expert on the cell biology and biochemistry of AT, Dr. Bohr will serve ChromaDex as an advisor through this process.
Dr. Bohr remarked, “This is a significant step towards providing treatment for AT, a disease with no cure. It is an honor to work as an advisor on such pivotal research, and we are committed to advancing the science behind NRC to meet the urgent needs of this rare disease community.”
“We are excited at the thought of embarking on these important future clinical trials for NRC as we are committed to advancing this to provide hope and relief for those suffering from this debilitating disease,” commented Dr. Susan Perlman, MD, Clinical Professor of Neurology and Director of the Ataxia Center at the UCLA Medical Center in Los Angeles, and ChromaDex Clinical Consultant.
For additional information on the science supporting NRC and for future updates visit www.chromadex.com .
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “will,” “should,” “could” or the negative of such terms or other similar expressions, and include the statements regarding the potential benefits and development of NRC as a treatment for AT or other diseases, including statements regarding clinical trials and obtaining IND Designation from the FDA. These forward-looking statements are based on the Company’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the ability to continue to pursue additional studies, human trials, and to obtain an IND Designation from the FDA; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov . Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
About ChromaDex:
ChromaDex Corp. (NASDAQ:CDXC) is the global authority on nicotinamide adenine dinucleotide (NAD + ), with a focus on the science of healthy aging. The ChromaDex team, comprised of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD + . A vital coenzyme found in every cell of the human body, NAD + declines with age and exposure to other everyday stressors. NAD + depletion is a contributor to age-related changes in health and vitality.
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
ChromaDex’s robust patent portfolio protects NR or nicotinamide riboside chloride (NRC) and other NAD + precursors. ChromaDex maintains a website at www.chromadex.com , to which ChromaDex regularly publishes copies of its press releases, news, and financial information.
ChromaDex Media Contact: Kendall Knysch, Senior Director of Media Relations & Partnerships 310-388-6706 ext. 689 [email protected]
ChromaDex Investor Relations Contact: Ben Shamsian Lytham Partners 646-829-9701 [email protected]
View source version on businesswire.com: https://www.businesswire.com/news/home/20240607042855/en/
Market Updates
What’s happening in the markets this week, what does nvidia’s stock split mean for investors, it’s been a terrible time for bonds. here’s why you should own them, which ai stocks are turning hype into revenue, best- and worst-performing stocks of may 2024, 3 stocks to buy and 3 stocks to sell in june, markets brief: friday’s job report in focus, these stocks are (still) powering the bull market, stock picks, nio earnings: revenue and loss largely in line, but vehicle margin missed amid price competition, the best biotech stocks to buy, after earnings, is marvell stock a buy, a sell, or fairly valued, nvidia raised its dividend — but there’s a catch, lululemon earnings: on track to meet expectations despite slowdown in americas, what is driving small-cap stock underperformance, after earnings, is zscaler stock a buy, a sell, or fairly valued, asml fair value raised as outlook improves, sponsor center.
- DOI: 10.1007/s00395-024-01042-4
- Corpus ID: 268689565
Insulin and glycolysis dependency of cardioprotection by nicotinamide riboside
- Y. Xiao , Q. Wang , +10 authors C. Zuurbier
- Published in Basic Research in Cardiology 25 March 2024
61 References
Cardioprotection by nicotinamide mononucleotide (nmn): involvement of glycolysis and acidic ph..
- Highly Influential
Nicotinamide Mononucleotide, an Intermediate of NAD+ Synthesis, Protects the Heart from Ischemia and Reperfusion
Nad+ metabolism in cardiac health, aging, and disease., nad+ and sirtuins in aging and disease., nicotinamide riboside promotes mfn2-mediated mitochondrial fusion in diabetic hearts through the sirt1-pgc1α-pparα pathway., cardiac metabolism as a driver and therapeutic target of myocardial infarction, insulin suppresses ischemic preconditioning-mediated cardioprotection through akt-dependent mechanisms., nlrx1 deletion increases ischemia-reperfusion damage and activates glucose metabolism in mouse heart, cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload, pharmacological cardioprotection against ischemia reperfusion injury—the search for a clinical effective therapy, related papers.
Showing 1 through 3 of 0 Related Papers
Become a member today, It's free!
Remember me
Send my password
Chromadex receives exclusive u.s. fda orphan drug designation (odd) and rare pediatric (rpd) disease designation for nicotinamide riboside chloride (nrc) for the treatment of ataxia telangiectasia (at), chromadex plans to conduct additional studies on nrc in anticipation of filing for an investigational new drug application (ind) for the treatment of at.
ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company’s product candidate for the treatment of Ataxia Telangiectasia (AT). Plans are underway to file an Investigational New Drug (IND) application with the U.S. FDA in anticipation of conducting human clinical trials, which will be guided by Dr. Vilhelm (Will) Bohr, Prof., University of Copenhagen and Scientific Advisor to ChromaDex.
“ Over 30 million people in the U.S. are impacted by more than 7,000 rare diseases, many of which are life-threatening and lack effective treatments,” said Rob Fried, CEO of ChromaDex. “We believe NRC has potential as a treatment for AT.”
“AT is a condition where children suffer from the adverse effects of premature aging and face a very limited life expectancy,” said Dr. Andrew Shao, ChromaDex Senior Vice President of Global Scientific & Regulatory Affairs. “We are excited to continue supporting the AT community and eagerly anticipate the results from future research.”
About Ataxia Telangiectasia (AT)
Ataxia Telangiectasia (AT) is a rare, progressive disease that typically presents in early childhood and is characterized by neurological and immunological symptoms. Those with AT often exhibit an unsteady gait (ataxia), impaired coordination of eye movements (oculomotor apraxia), and involuntary movements (choreoathetosis). AT leads to cerebellar degeneration and many affected children become wheelchair-dependent. Currently, there is no cure or FDA-approved treatment to slow the progression of AT, with the average life expectancy being around 25 years for those diagnosed in childhood.
There are many types of ataxia. Another form of ataxia is Friedreich’s ataxia (FA), which is being addressed by Biogen and Larimar Therapeutics. FA impacts 1 in 50,000 people in the U.S. ( NIH ). Ataxia Telangiectasia (AT) impacts roughly 1 in 40,000 people in the U.S. (Riboldi et al., 2023; Tieve et al., 2015).
Clinical Research on NRC and AT
To date, NRC has been investigated in two third-party funded, peer-reviewed published clinical trials for the treatment of AT. The first study published in Movement Disorders demonstrated that supplementation with NRC improved AT scores and increased immunoglobulins, or antibodies, in the immune-compromised patients, with AT score improvements reversing once supplementation concluded (Veenhius et al., 2021).
The second phase II two-year long study, also published in Movement Disorders , demonstrated that long-term NRC supplementation increased whole blood NAD + levels up to fourfold, and improved neuromotor coordination and eye movements in 90% of participants while maintaining biomarkers of stable liver and kidney function, as compared to historical disease progression (Presterud et al., 2023). Both studies reported no serious adverse events, with NRC being generally well-tolerated.
Not associated with ChromaDex’s future NRC IND filing, there are two additional investigator-initiated ongoing registered clinical trials that will examine NR supplementation in AT patients. The first is a continuation of Presterud et al. 2023 and will track AT patients over the course of 8-10 years. Recently registered, the second will be a single-arm open-label clinical trial scheduled to commence this year in Australia.
Significance of Orphan Drug Designation (OOD) and Rare Pediatric Disease Designation (RPD)
According to the FDA , there are too few treatments for rare diseases because of high research and development costs, which companies often cannot recoup as a result of small patient populations. To incentivize companies to invest in bringing treatments to market for rare diseases, in 1983, Congress passed the Orphan Drug Act, which makes Orphan Drug Designation (ODD) candidates eligible for tax credits, waives their user fees, and may provide a period of exclusivity should the orphan drug be subsequently approved by FDA.
Related to ODD, the FDA’s Rare Pediatric Disease (RPD) designation , further incentivizes companies to invest in rare childhood diseases by providing a voucher program to applicants approved by September 30th, 2024. Through this program, companies with RPD designation that ultimately obtain successful drug approval for a rare pediatric disease are provided a voucher, which can be used to expedite the FDA review of another drug candidate or sold to other companies.
Future Clinical Trials on NRC and AT
With both designations granted, ChromaDex plans to file an Investigational New Drug (IND) application for future human clinical trials for the use of NRC in the treatment of AT. As a leading expert on the cell biology and biochemistry of AT, Dr. Bohr will serve ChromaDex as an advisor through this process.
Dr. Bohr remarked, “This is a significant step towards providing treatment for AT, a disease with no cure. It is an honor to work as an advisor on such pivotal research, and we are committed to advancing the science behind NRC to meet the urgent needs of this rare disease community.”
“We are excited at the thought of embarking on these important future clinical trials for NRC as we are committed to advancing this to provide hope and relief for those suffering from this debilitating disease,” commented Dr. Susan Perlman, MD, Clinical Professor of Neurology and Director of the Ataxia Center at the UCLA Medical Center in Los Angeles, and ChromaDex Clinical Consultant.
For additional information on the science supporting NRC and for future updates visit www.chromadex.com .
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “will,” “should,” “could” or the negative of such terms or other similar expressions, and include the statements regarding the potential benefits and development of NRC as a treatment for AT or other diseases, including statements regarding clinical trials and obtaining IND Designation from the FDA. These forward-looking statements are based on the Company’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the ability to continue to pursue additional studies, human trials, and to obtain an IND Designation from the FDA; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov . Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
About ChromaDex:
ChromaDex Corp. (NASDAQ:CDXC) is the global authority on nicotinamide adenine dinucleotide (NAD + ), with a focus on the science of healthy aging. The ChromaDex team, comprised of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD + . A vital coenzyme found in every cell of the human body, NAD + declines with age and exposure to other everyday stressors. NAD + depletion is a contributor to age-related changes in health and vitality.
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
ChromaDex’s robust patent portfolio protects NR or nicotinamide riboside chloride (NRC) and other NAD + precursors. ChromaDex maintains a website at www.chromadex.com , to which ChromaDex regularly publishes copies of its press releases, news, and financial information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240607042855/en/
Related News
@ the bell: despite struggles, tsx ends week steady, @ the bell: resource stocks lift tsx, @ the bell: how did markets react to the boc rate cut, recent u.s. press releases, mutual capital group to acquire icc holdings, inc. for $23.50 per share in $73.8 million..., virgin galactic completes 12th successful spaceflight, deadline in 9 days: kessler topaz meltzer & check, llp reminds investors of deadline in class..., featured news links, thematic insights: free podcast exploring market trends & emerging sectors, flow test and laboratory analysis confirm a major new helium discovery in minnesota, this small-cap gold stock keeps notching up high-grade gold in nevada.
Get the latest news and updates from Stockhouse on social media
Follow STOCKHOUSE Today

We could not find any results for: Make sure your spelling is correct or try broadening your search.
Trending Now

It looks like you aren't logged in. Click the button below to log in and view your recent history.
Already a member? Sign in
Interactive Brokers


Market Data
- Latest News
- News Scanner
- News Alerts
Trading Tools
- Economic Calendar
- Trader Alerts
- Price Alerts
Follow Feed
- Currency Converter
- Commodities
- World Exchanges
- Market Movers
Options Flow
- Bitcoin ETFs
- Broker Review
- Global Interest Rates
Hot Features
Connect with traders and investors in our Follow Feed community.
Explore comprehensive options data and use advanced filters with Options Flow.
Organize and monitor your stock and asset watchlist with Monitor.
- 1-888-992-3836
- [email protected]
- United States
- United Kingdom
- Deutschland
- México
- 日本
- 대한민국
CDXC ChromaDex Corporation
ChromaDex plans to conduct additional studies on NRC in anticipation of filing for an Investigational New Drug Application (IND) for the treatment of AT
ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company’s product candidate for the treatment of Ataxia Telangiectasia (AT). Plans are underway to file an Investigational New Drug (IND) application with the U.S. FDA in anticipation of conducting human clinical trials, which will be guided by Dr. Vilhelm (Will) Bohr, Prof., University of Copenhagen and Scientific Advisor to ChromaDex.
“Over 30 million people in the U.S. are impacted by more than 7,000 rare diseases, many of which are life-threatening and lack effective treatments,” said Rob Fried, CEO of ChromaDex. “We believe NRC has potential as a treatment for AT.”
“AT is a condition where children suffer from the adverse effects of premature aging and face a very limited life expectancy,” said Dr. Andrew Shao, ChromaDex Senior Vice President of Global Scientific & Regulatory Affairs. “We are excited to continue supporting the AT community and eagerly anticipate the results from future research.”
About Ataxia Telangiectasia (AT)
Ataxia Telangiectasia (AT) is a rare, progressive disease that typically presents in early childhood and is characterized by neurological and immunological symptoms. Those with AT often exhibit an unsteady gait (ataxia), impaired coordination of eye movements (oculomotor apraxia), and involuntary movements (choreoathetosis). AT leads to cerebellar degeneration and many affected children become wheelchair-dependent. Currently, there is no cure or FDA-approved treatment to slow the progression of AT, with the average life expectancy being around 25 years for those diagnosed in childhood.
There are many types of ataxia. Another form of ataxia is Friedreich’s ataxia (FA), which is being addressed by Biogen and Larimar Therapeutics. FA impacts 1 in 50,000 people in the U.S. ( NIH ). Ataxia Telangiectasia (AT) impacts roughly 1 in 40,000 people in the U.S. (Riboldi et al., 2023; Tieve et al., 2015).
Clinical Research on NRC and AT
To date, NRC has been investigated in two third-party funded, peer-reviewed published clinical trials for the treatment of AT. The first study published in Movement Disorders demonstrated that supplementation with NRC improved AT scores and increased immunoglobulins, or antibodies, in the immune-compromised patients, with AT score improvements reversing once supplementation concluded (Veenhius et al., 2021).
The second phase II two-year long study, also published in Movement Disorders , demonstrated that long-term NRC supplementation increased whole blood NAD + levels up to fourfold, and improved neuromotor coordination and eye movements in 90% of participants while maintaining biomarkers of stable liver and kidney function, as compared to historical disease progression (Presterud et al., 2023). Both studies reported no serious adverse events, with NRC being generally well-tolerated.
Not associated with ChromaDex’s future NRC IND filing, there are two additional investigator-initiated ongoing registered clinical trials that will examine NR supplementation in AT patients. The first is a continuation of Presterud et al. 2023 and will track AT patients over the course of 8-10 years. Recently registered, the second will be a single-arm open-label clinical trial scheduled to commence this year in Australia.
Significance of Orphan Drug Designation (OOD) and Rare Pediatric Disease Designation (RPD)
According to the FDA, there are too few treatments for rare diseases because of high research and development costs, which companies often cannot recoup as a result of small patient populations. To incentivize companies to invest in bringing treatments to market for rare diseases, in 1983, Congress passed the Orphan Drug Act, which makes Orphan Drug Designation (ODD) candidates eligible for tax credits, waives their user fees, and may provide a period of exclusivity should the orphan drug be subsequently approved by FDA.
Related to ODD, the FDA’s Rare Pediatric Disease (RPD) designation, further incentivizes companies to invest in rare childhood diseases by providing a voucher program to applicants approved by September 30th, 2024. Through this program, companies with RPD designation that ultimately obtain successful drug approval for a rare pediatric disease are provided a voucher, which can be used to expedite the FDA review of another drug candidate or sold to other companies.
Future Clinical Trials on NRC and AT
With both designations granted, ChromaDex plans to file an Investigational New Drug (IND) application for future human clinical trials for the use of NRC in the treatment of AT. As a leading expert on the cell biology and biochemistry of AT, Dr. Bohr will serve ChromaDex as an advisor through this process.
Dr. Bohr remarked, “This is a significant step towards providing treatment for AT, a disease with no cure. It is an honor to work as an advisor on such pivotal research, and we are committed to advancing the science behind NRC to meet the urgent needs of this rare disease community.”
“We are excited at the thought of embarking on these important future clinical trials for NRC as we are committed to advancing this to provide hope and relief for those suffering from this debilitating disease,” commented Dr. Susan Perlman, MD, Clinical Professor of Neurology and Director of the Ataxia Center at the UCLA Medical Center in Los Angeles, and ChromaDex Clinical Consultant.
For additional information on the science supporting NRC and for future updates visit www.chromadex.com.
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “will,” “should,” “could” or the negative of such terms or other similar expressions, and include the statements regarding the potential benefits and development of NRC as a treatment for AT or other diseases, including statements regarding clinical trials and obtaining IND Designation from the FDA. These forward-looking statements are based on the Company’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the ability to continue to pursue additional studies, human trials, and to obtain an IND Designation from the FDA; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
About ChromaDex:
ChromaDex Corp. (NASDAQ:CDXC) is the global authority on nicotinamide adenine dinucleotide (NAD + ), with a focus on the science of healthy aging. The ChromaDex team, comprised of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD + . A vital coenzyme found in every cell of the human body, NAD + declines with age and exposure to other everyday stressors. NAD + depletion is a contributor to age-related changes in health and vitality.
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
ChromaDex’s robust patent portfolio protects NR or nicotinamide riboside chloride (NRC) and other NAD + precursors. ChromaDex maintains a website at www.chromadex.com, to which ChromaDex regularly publishes copies of its press releases, news, and financial information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240607042855/en/
ChromaDex Media Contact: Kendall Knysch, Senior Director of Media Relations & Partnerships 310-388-6706 ext. 689 [email protected]
ChromaDex Investor Relations Contact: Ben Shamsian Lytham Partners 646-829-9701 [email protected]
Latest CDXC Messages
| {{bbMessage.MSG_Date}} {{bbMessage.HowLongAgo}} {{bbMessage.MSG_ID}} Share on Facebook Share on Twitter Share on Email |
Loading Messages....
Chromadex corporation news.
More ChromaDex Corporation News Articles
Your Recent History
It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
- Investor Relations
- Advertise With Us
- Broker Listing
- Data Accreditations
- Consent Preferences
Get The App
Support: 1-888-992-3836 | [email protected]
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions
- Cookie and Privacy Policy
- Investment Warning
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
- Investor Relations
News Details
Chromadex receives exclusive u.s. fda orphan drug designation (odd) and rare pediatric (rpd) disease designation for nicotinamide riboside chloride (nrc) for the treatment of ataxia telangiectasia (at).
ChromaDex plans to conduct additional studies on NRC in anticipation of filing for an Investigational New Drug Application (IND) for the treatment of AT
LOS ANGELES--(BUSINESS WIRE)-- ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company’s product candidate for the treatment of Ataxia Telangiectasia (AT). Plans are underway to file an Investigational New Drug (IND) application with the U.S. FDA in anticipation of conducting human clinical trials, which will be guided by Dr. Vilhelm (Will) Bohr, Prof., University of Copenhagen and Scientific Advisor to ChromaDex.
“ Over 30 million people in the U.S. are impacted by more than 7,000 rare diseases, many of which are life-threatening and lack effective treatments,” said Rob Fried, CEO of ChromaDex. “We believe NRC has potential as a treatment for AT.”
“AT is a condition where children suffer from the adverse effects of premature aging and face a very limited life expectancy,” said Dr. Andrew Shao, ChromaDex Senior Vice President of Global Scientific & Regulatory Affairs. “We are excited to continue supporting the AT community and eagerly anticipate the results from future research.”
About Ataxia Telangiectasia (AT)
Ataxia Telangiectasia (AT) is a rare, progressive disease that typically presents in early childhood and is characterized by neurological and immunological symptoms. Those with AT often exhibit an unsteady gait (ataxia), impaired coordination of eye movements (oculomotor apraxia), and involuntary movements (choreoathetosis). AT leads to cerebellar degeneration and many affected children become wheelchair-dependent. Currently, there is no cure or FDA-approved treatment to slow the progression of AT, with the average life expectancy being around 25 years for those diagnosed in childhood.
There are many types of ataxia. Another form of ataxia is Friedreich’s ataxia (FA), which is being addressed by Biogen and Larimar Therapeutics. FA impacts 1 in 50,000 people in the U.S. ( NIH ). Ataxia Telangiectasia (AT) impacts roughly 1 in 40,000 people in the U.S. (Riboldi et al., 2023; Tieve et al., 2015).
Clinical Research on NRC and AT
To date, NRC has been investigated in two third-party funded, peer-reviewed published clinical trials for the treatment of AT. The first study published in Movement Disorders demonstrated that supplementation with NRC improved AT scores and increased immunoglobulins, or antibodies, in the immune-compromised patients, with AT score improvements reversing once supplementation concluded (Veenhius et al., 2021).
The second phase II two-year long study, also published in Movement Disorders , demonstrated that long-term NRC supplementation increased whole blood NAD + levels up to fourfold, and improved neuromotor coordination and eye movements in 90% of participants while maintaining biomarkers of stable liver and kidney function, as compared to historical disease progression (Presterud et al., 2023). Both studies reported no serious adverse events, with NRC being generally well-tolerated.
Not associated with ChromaDex’s future NRC IND filing, there are two additional investigator-initiated ongoing registered clinical trials that will examine NR supplementation in AT patients. The first is a continuation of Presterud et al. 2023 and will track AT patients over the course of 8-10 years. Recently registered, the second will be a single-arm open-label clinical trial scheduled to commence this year in Australia.
Significance of Orphan Drug Designation (OOD) and Rare Pediatric Disease Designation (RPD)
According to the FDA , there are too few treatments for rare diseases because of high research and development costs, which companies often cannot recoup as a result of small patient populations. To incentivize companies to invest in bringing treatments to market for rare diseases, in 1983, Congress passed the Orphan Drug Act, which makes Orphan Drug Designation (ODD) candidates eligible for tax credits, waives their user fees, and may provide a period of exclusivity should the orphan drug be subsequently approved by FDA.
Related to ODD, the FDA’s Rare Pediatric Disease (RPD) designation , further incentivizes companies to invest in rare childhood diseases by providing a voucher program to applicants approved by September 30th, 2024. Through this program, companies with RPD designation that ultimately obtain successful drug approval for a rare pediatric disease are provided a voucher, which can be used to expedite the FDA review of another drug candidate or sold to other companies.
Future Clinical Trials on NRC and AT
With both designations granted, ChromaDex plans to file an Investigational New Drug (IND) application for future human clinical trials for the use of NRC in the treatment of AT. As a leading expert on the cell biology and biochemistry of AT, Dr. Bohr will serve ChromaDex as an advisor through this process.
Dr. Bohr remarked, “This is a significant step towards providing treatment for AT, a disease with no cure. It is an honor to work as an advisor on such pivotal research, and we are committed to advancing the science behind NRC to meet the urgent needs of this rare disease community.”
“We are excited at the thought of embarking on these important future clinical trials for NRC as we are committed to advancing this to provide hope and relief for those suffering from this debilitating disease,” commented Dr. Susan Perlman, MD, Clinical Professor of Neurology and Director of the Ataxia Center at the UCLA Medical Center in Los Angeles, and ChromaDex Clinical Consultant.
For additional information on the science supporting NRC and for future updates visit www.chromadex.com .
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “will,” “should,” “could” or the negative of such terms or other similar expressions, and include the statements regarding the potential benefits and development of NRC as a treatment for AT or other diseases, including statements regarding clinical trials and obtaining IND Designation from the FDA. These forward-looking statements are based on the Company’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the ability to continue to pursue additional studies, human trials, and to obtain an IND Designation from the FDA; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov . Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
About ChromaDex:
ChromaDex Corp. (NASDAQ:CDXC) is the global authority on nicotinamide adenine dinucleotide (NAD + ), with a focus on the science of healthy aging. The ChromaDex team, comprised of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD + . A vital coenzyme found in every cell of the human body, NAD + declines with age and exposure to other everyday stressors. NAD + depletion is a contributor to age-related changes in health and vitality.
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
ChromaDex’s robust patent portfolio protects NR or nicotinamide riboside chloride (NRC) and other NAD + precursors. ChromaDex maintains a website at www.chromadex.com , to which ChromaDex regularly publishes copies of its press releases, news, and financial information.
ChromaDex Media Contact: Kendall Knysch, Senior Director of Media Relations & Partnerships 310-388-6706 ext. 689 [email protected]
ChromaDex Investor Relations Contact: Ben Shamsian Lytham Partners 646-829-9701 [email protected]

Investor Relations Contact
Ben Shamsian Lytham Partners 646-829-9701 [email protected]
+1- 949-356-1620 [email protected]
Mike Moyer, LifeSci Advisors, Managing Director +1-617-308-4306 [email protected]
Investor Email Alerts
To opt-in for investor email alerts, please enter your email address in the field below and select at least one alert option. After submitting your request, you will receive an activation email to the requested email address. You must click the activation link in order to complete your subscription. You can sign up for additional alert options at any time.
At ChromaDex Corp., we promise to treat your data with respect and will not share your information with any third party. You can unsubscribe to any of the investor alerts you are subscribed to by visiting the ‘unsubscribe’ section below. If you experience any issues with this process, please contact us for further assistance.
By providing your email address below, you are providing consent to ChromaDex Corp. to send you the requested Investor Email Alert updates.
| * |
| * |
| |
Email Alert Sign Up Confirmation
- Press Releases
- Privacy Policy
- California Consumer Privacy Statement
- Terms of Use
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Biden Economy
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit Cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Student Loans
- Personal Loans
- Car Insurance
- Mortgage Calculator
- Morning Brief
- Market Domination
- Market Domination Overtime
- Asking for a Trend
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Chromadex receives exclusive u.s. fda orphan drug designation (odd) and rare pediatric (rpd) disease designation for nicotinamide riboside chloride (nrc) for the treatment of ataxia telangiectasia (at).
ChromaDex plans to conduct additional studies on NRC in anticipation of filing for an Investigational New Drug Application (IND) for the treatment of AT
LOS ANGELES, June 07, 2024 --( BUSINESS WIRE )-- ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company’s product candidate for the treatment of Ataxia Telangiectasia (AT). Plans are underway to file an Investigational New Drug (IND) application with the U.S. FDA in anticipation of conducting human clinical trials, which will be guided by Dr. Vilhelm (Will) Bohr, Prof., University of Copenhagen and Scientific Advisor to ChromaDex.
" Over 30 million people in the U.S. are impacted by more than 7,000 rare diseases, many of which are life-threatening and lack effective treatments," said Rob Fried, CEO of ChromaDex. "We believe NRC has potential as a treatment for AT."
"AT is a condition where children suffer from the adverse effects of premature aging and face a very limited life expectancy," said Dr. Andrew Shao, ChromaDex Senior Vice President of Global Scientific & Regulatory Affairs. "We are excited to continue supporting the AT community and eagerly anticipate the results from future research."
About Ataxia Telangiectasia (AT)
Ataxia Telangiectasia (AT) is a rare, progressive disease that typically presents in early childhood and is characterized by neurological and immunological symptoms. Those with AT often exhibit an unsteady gait (ataxia), impaired coordination of eye movements (oculomotor apraxia), and involuntary movements (choreoathetosis). AT leads to cerebellar degeneration and many affected children become wheelchair-dependent. Currently, there is no cure or FDA-approved treatment to slow the progression of AT, with the average life expectancy being around 25 years for those diagnosed in childhood.
There are many types of ataxia. Another form of ataxia is Friedreich’s ataxia (FA), which is being addressed by Biogen and Larimar Therapeutics. FA impacts 1 in 50,000 people in the U.S. ( NIH ). Ataxia Telangiectasia (AT) impacts roughly 1 in 40,000 people in the U.S. (Riboldi et al., 2023; Tieve et al., 2015).
Clinical Research on NRC and AT
To date, NRC has been investigated in two third-party funded, peer-reviewed published clinical trials for the treatment of AT. The first study published in Movement Disorders demonstrated that supplementation with NRC improved AT scores and increased immunoglobulins, or antibodies, in the immune-compromised patients, with AT score improvements reversing once supplementation concluded (Veenhius et al., 2021).
The second phase II two-year long study, also published in Movement Disorders , demonstrated that long-term NRC supplementation increased whole blood NAD + levels up to fourfold, and improved neuromotor coordination and eye movements in 90% of participants while maintaining biomarkers of stable liver and kidney function, as compared to historical disease progression (Presterud et al., 2023). Both studies reported no serious adverse events, with NRC being generally well-tolerated.
Not associated with ChromaDex’s future NRC IND filing, there are two additional investigator-initiated ongoing registered clinical trials that will examine NR supplementation in AT patients. The first is a continuation of Presterud et al. 2023 and will track AT patients over the course of 8-10 years. Recently registered, the second will be a single-arm open-label clinical trial scheduled to commence this year in Australia.
Significance of Orphan Drug Designation (OOD) and Rare Pediatric Disease Designation (RPD)
According to the FDA , there are too few treatments for rare diseases because of high research and development costs, which companies often cannot recoup as a result of small patient populations. To incentivize companies to invest in bringing treatments to market for rare diseases, in 1983, Congress passed the Orphan Drug Act, which makes Orphan Drug Designation (ODD) candidates eligible for tax credits, waives their user fees, and may provide a period of exclusivity should the orphan drug be subsequently approved by FDA.
Related to ODD, the FDA’s Rare Pediatric Disease (RPD) designation , further incentivizes companies to invest in rare childhood diseases by providing a voucher program to applicants approved by September 30th, 2024. Through this program, companies with RPD designation that ultimately obtain successful drug approval for a rare pediatric disease are provided a voucher, which can be used to expedite the FDA review of another drug candidate or sold to other companies.
Future Clinical Trials on NRC and AT
With both designations granted, ChromaDex plans to file an Investigational New Drug (IND) application for future human clinical trials for the use of NRC in the treatment of AT. As a leading expert on the cell biology and biochemistry of AT, Dr. Bohr will serve ChromaDex as an advisor through this process.
Dr. Bohr remarked, "This is a significant step towards providing treatment for AT, a disease with no cure. It is an honor to work as an advisor on such pivotal research, and we are committed to advancing the science behind NRC to meet the urgent needs of this rare disease community."
"We are excited at the thought of embarking on these important future clinical trials for NRC as we are committed to advancing this to provide hope and relief for those suffering from this debilitating disease," commented Dr. Susan Perlman, MD, Clinical Professor of Neurology and Director of the Ataxia Center at the UCLA Medical Center in Los Angeles, and ChromaDex Clinical Consultant.
For additional information on the science supporting NRC and for future updates visit www.chromadex.com .
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as "expects," "anticipates," "intends," "estimates," "plans," "potential," "possible," "probable," "believes," "seeks," "may," "will," "should," "could" or the negative of such terms or other similar expressions, and include the statements regarding the potential benefits and development of NRC as a treatment for AT or other diseases, including statements regarding clinical trials and obtaining IND Designation from the FDA. These forward-looking statements are based on the Company’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the ability to continue to pursue additional studies, human trials, and to obtain an IND Designation from the FDA; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov . Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
About ChromaDex:
ChromaDex Corp. (NASDAQ:CDXC) is the global authority on nicotinamide adenine dinucleotide (NAD + ), with a focus on the science of healthy aging. The ChromaDex team, comprised of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD + . A vital coenzyme found in every cell of the human body, NAD + declines with age and exposure to other everyday stressors. NAD + depletion is a contributor to age-related changes in health and vitality.
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
ChromaDex’s robust patent portfolio protects NR or nicotinamide riboside chloride (NRC) and other NAD + precursors. ChromaDex maintains a website at www.chromadex.com , to which ChromaDex regularly publishes copies of its press releases, news, and financial information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240607042855/en/
ChromaDex Media Contact: Kendall Knysch, Senior Director of Media Relations & Partnerships 310-388-6706 ext. 689 [email protected]
ChromaDex Investor Relations Contact: Ben Shamsian Lytham Partners 646-829-9701 [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 July 2019
Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults
- Dietrich Conze 1 na1 ,
- Charles Brenner ORCID: orcid.org/0000-0002-4955-3226 2 &
- Claire L. Kruger 1 na1
Scientific Reports volume 9 , Article number: 9772 ( 2019 ) Cite this article
132k Accesses
122 Citations
346 Altmetric
Metrics details
- Diagnostic markers
- Drug safety
Nicotinamide riboside (NR) is a newly discovered nicotinamide adenine dinucleotide (NAD + ) precursor vitamin. A crystal form of NR chloride termed NIAGEN is generally recognized as safe (GRAS) for use in foods and the subject of two New Dietary Ingredient Notifications for use in dietary supplements. To evaluate the kinetics and dose-dependency of NR oral availability and safety in overweight, but otherwise healthy men and women, an 8-week randomized, double-blind, placebo-controlled clinical trial was conducted. Consumption of 100, 300 and 1000 mg NR dose-dependently and significantly increased whole blood NAD + (i.e., 22%, 51% and 142%) and other NAD + metabolites within 2 weeks. The increases were maintained throughout the remainder of the study. There were no reports of flushing and no significant differences in adverse events between the NR and placebo-treated groups or between groups at different NR doses. NR also did not elevate low density lipoprotein cholesterol or dysregulate 1-carbon metabolism. Together these data support the development of a tolerable upper intake limit for NR based on human data.
Similar content being viewed by others
Safety evaluation of β-nicotinamide mononucleotide oral administration in healthy adult men and women
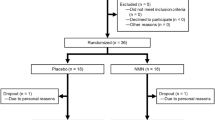
Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial

Safety and tolerability of high-dose daily vitamin D3 supplementation in the vitamin D and type 2 diabetes (D2d) study—a randomized trial in persons with prediabetes
Introduction.
The NAD + co-enzymes NAD + , NADH, NADP + and NADPH are the central regulators of metabolism. They are required for fuel oxidation, ATP generation, gluconeogenesis, ketogenesis, production of pentose phosphates, heme, lipids, steroid hormones and detoxification of free radical species 1 , 2 . NAD + is also a consumed substrate of enzymes that polymerize and/or transfer ADPribose, form cyclic ADPribose (cyclic ADPribose synthetases) and deacylate protein lysine substrates (sirtuins) with production of acyl-ADPribosyl products. Poly(ADPribose) polymerases (PARPs) signal DNA damage in order to assemble repair machinery, while cyclic ADPribose synthetases produce second messengers that mobilize calcium ions from intracellular stores, and sirtuins influence gene expression and protein activities by virtue of reversing protein post-translational modifications 3 . In light of the important roles of NAD + co-enzymes in metabolism and mediating some of the longevity benefits of calorie restriction via sirtuins, there is a renewed interest in the synthesis and maintenance of the NAD + metabolome 4 .
All tissues produce NAD + from nicotinamide (NAM) or the recently identified NAD + precursor, nicotinamide riboside (NR) 5 Some tissues can produce NAD + from tryptophan de novo and nicotinic acid (NA) 2 , although the generation of NAD + from tryptophan is much less efficient than from the vitamin precursors of NA, NAM, or NR, which are collectively termed vitamin B3. NAD + can also be supported by dietary precursors 6 . For example, pellagra, a disease of deficiency of NAD + precursors, can be prevented or treated with approximately 15 mg/day of NA or NAM or with 60-times as much tryptophan 7 . Importantly, despite homeostatic systems and dietary intake of NAD + precursors, it is now known that the levels of NAD + co-enzymes are continuously challenged by metabolic stress. In the overfed and type 2 diabetic mouse livers, levels of NADPH are strikingly depressed 8 , whereas in noise-induced hearing loss 9 , heart failure 10 , peripheral nerve damage 11 , central brain injury 12 and the liver of a lactating mouse 13 , NAD + levels are compromised. Moreover, NAD + levels have been reported to decline in response to DNA damage 14 , alcohol metabolism 15 , and aging 16 , 17 , and the expression of nicotinamide phosphoribosyltransferase (NAMPT), the enzyme required for NAM salvage, declines with aging 18 and chronic inflammation 19 . Thus, considering the relationships between NAD + , metabolic stress and aging, nutritional scientists are now investigating whether the ingestion of higher levels of a B3 vitamin should be part of an evidence-based approach to optimize health 2 .
Although NA, NAM, and NR all produce NAD + and NADP + 2 , 7 , 20 , it is important to note that each precursor has unique effects physiologically. NA can lower blood lipids and is used to treat dyslipidemia 21 . At doses of greater than 50 mg/day, NA can also induce flushing 6 , 21 . In contrast, NAM does not lower blood lipids or cause flushing, has been reported be a sirtuin inhibitor at high doses 20 , 22 , and appears to have a greater effect at elevating blood levels of homocysteine (HCY) in humans than NA via its metabolism to 1-methylnicotinamide (MeNAM) 23 . In yeast, NR activates SIR2 and extends replicative lifespan 24 . In mouse models, NR prevents high-fat diet-induced weight gain 25 , fatty liver and diabetic peripheral neuropathy 8 , noise-induced hearing loss 9 , heart failure 10 , and central brain injury 12 . In addition, oral NR greatly improves survival and hematopoietic stem cell regeneration after irradiation of mice—an activity that was not seen in NA or NAM supplemented mice 26 . In rats, oral NR promotes resistance to and reversal of chemotherapeutic neuropathy 27 . In mice, oral NR increases the hepatic levels of the NAD + metabolome with pharmacokinetics that are superior to that of NA and NAM 20 . In addition, postpartum female mice and rats who were administered NR exhibited increased lactation and produced offspring that are stronger, less anxious, have better memory, and have enhanced adult hippocampal neurogenesis and body composition as adults 13 . Because NR does not cause flushing or inhibit sirtuins 25 and the genes (NRK1 and NRK2) required for the metabolism of NR to NAD + are upregulated in conditions of metabolic stress 10 , 28 , NR has a particularly strong potential as a distinct vitamin B3 to support human wellness during metabolic stress and aging.
In a variety of animal models, nicotinamide mononucleotide (NMN), the 5′-phosphorylated form of NR, has also shown promise in conditions of metabolic stress and aging 29 . Moreover, the gut-expressed multispanning membrane protein Slc12a8, previously annotated as a Na + /K + Cl − transporter, has been proposed to be a specific transporter of nicotinamide mononucleotide (NMN) 30 . However, the assignment of Slc12a8 as a transporter of NMN occurred without a reliable LC-tandem MS assay for the expected concentration of NMN 31 and are inconsistent with genetic, cell biological, and pharmacological evidence from multiple studies demonstrating that NMN is extracellularly converted to NAM and NR prior to intracellular conversion to NMN and the rest of the NAD metabolome 12 , 32 , 33 , 34 , 35 , 36 . While it remains possible that data will emerge showing convincing NMN transport in one or more tissues, the consensus view is that NMN is a usefully circulating metabolite that makes NR available at plasma membranes, which express the 5′-nucleotidase activity of CD73 1 , 34 . To our knowledge, tests of the safety and human oral availability of NMN are not yet available.
A crystalline form of NR chloride termed NIAGEN has been evaluated in a battery of preclinical studies including a bacterial reverse mutagenesis assay, an in vitro chromosome aberration assay, an in vivo micronucleus assay, and acute, 14-day and 90-day rat toxicology 37 . In the 90-day toxicology study, NR had a similar toxicity profile to NAM at equimolar doses, the lowest observed adverse effect level (LOAEL) for NR was 1000 mg/kg/day, and the no observed adverse effect level (NOAEL) was 300 mg/kg/day. NIAGEN is Generally Recognized as Safe (GRAS) in the United States for use in food products 38 and the subject of two new dietary ingredient notifications 39 , 40 , which were filed with the United States Food and Drug Administration without objection.
To date, NR has also been tested in six clinical trials. The first clinical trial of NR established the safe oral availability of single doses and the timecourse by which NR elevates the human blood NAD metabolome 20 . The second trial provided additional safety data for healthy people taking NR for 8 days 41 . The third and fourth trials addressed NR safety in healthy people either taking 500 mg NR twice daily for 6 weeks or combination of up to 500 mg NR and 100 mg pterostilbene per day for 8 weeks 42 , 43 . Whereas Dellinger et al . 42 found that the combination of NR and pterostilbene signficantly elevated low density protein cholesterol (LDL-C) in a dose and time-depended fashion 42 , no signficant increases in LDL-C were seen following the adminstration of NR alone 43 . A fifth clinical trial documented the safety and tolerance of ingesting 2 grams NR per day for 12 weeks in obese men and post hoc analyses suggested that there was an improvement in fatty liver in the NR-treated group 44 . In a sixth clinical trial, single 500 mg doses of NR depressed markers of oxidative damage while increasing NADPH and exercise performance in older individuals 45 .
To address the dose-dependent oral availability and safety of NR in overweight adults and the safety of daily NR without pterostilbene including effects on LDL-C and blood levels of HCY, we conducted a randomized, 8-week placebo-controlled trial with 3 doses of NR in overweight but otherwise healthy adults. Here we show that once a day doses of NR up to 1 gram per day are safe and orally available. Blood NAD + was increased in study subjects in a dose-dependent manner with NAD + levels achieving 14% to 114% increased levels within 2 weeks that were sustained. We also establish that daily high dose ingestion of NR does not elevate LDL-C or plasma HCY.
Study design
One hundred and forty healthy male and female participants were enrolled in a randomized, double-blind, placebo-controlled parallel study to investigate the safety and effect of NR (100 mg/day, 300 mg/day, and 1000 mg/day) on NAD + metabolite concentrations in urine and blood over 8 weeks. The study consisted of a 2-week run-in and 8-week supplementation period (Fig. 1 ). To minimize the effect of dietary influences on NAD + metabolite levels, subjects were instructed to avoid foods that contain high amounts of tryptophan and forms of vitamin B3 during the run-in and NR supplementation periods. After screening, all subjects attended the clinic prior to the run-in period to review their medical history and health status and receive counseling for the dietary restrictions. At the end of the run-in period (Day 0), the subjects visited the clinic for baseline safety assessments, blood and urine collection, randomization to one of four supplementation groups (placebo, 100 mg, 300 mg, 1000 mg NIAGEN per day groups; n = 35/group), and additional dietary restriction counseling. The subjects were then released to consume their study product for the subsequent 56 days, attending the clinic on Day 7, 14, 28, and 56 for safety assessments, and blood and urine collection. The study was conducted at KGK Science Inc. Suite 1440, One London Place, 255 Queens Ave, London, Ontario, following Good Clinical Practice (GCP) guidelines and in accordance with the ethical principles that have their origins in the Declaration of Helsinki and its subsequent amendments. The study was reviewed by the Natural Health Product Directorate (NHPD), Health Canada and a research ethics board. Notice of authorization was granted on December 9 th , 2015 by the NHPD, Ottawa, Ontario and unconditional approval was granted on February 5 th , 2016 by the Institutional Review Board (IRB Services, Aurora, Ontario). The study was registered on clinicaltrials.org on March 18, 2016 as NCT0271593 and posted to the WHO International Clinical Trial Registry Platform on January 3, 2016. External monitoring of source documents was conducted by ClynProject Consulting, LLC.
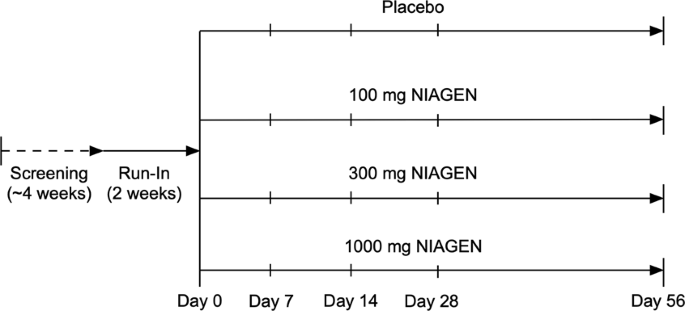
Study design. Subjects were screened over a 4-week period. Eligible subjects were enrolled and instructed to avoid foods containing high amounts of tryptophan and forms of niacin for the duration of the study. Following a 2-week run-in period, the subjects visited the clinic on Day 0 for baseline safety assessments, blood and urine collection, and randomization to one of four supplementation groups (placebo, 100 mg, 300 mg, 1000 mg NIAGEN per day). The subjects then consumed either placebo or the NIAGEN treatments for 56 days and visited the clinic on Day 7, 14, 28, and 56 for safety assessments, and blood and urine collection. Dietary counseling and food records were dispensed and collected throughout the run-in and supplementation periods to ensure that the subjects adhered to the dietary restrictions.
The primary objective was to evaluate the difference in urinary MeNAM levels between placebo and NIAGEN (100 mg, 300 mg, and 1000 mg) after 8 weeks of supplementation. The secondary objectives were to evaluate the rate of increase in urinary MeNAM levels between placebo and NIAGEN (100 mg, 300 mg, and 1000 mg) after 8 weeks of supplementation, the difference and rate of increase in other NR metabolites levels in blood between placebo and NIAGEN (100 mg, 300 mg, and 1000 mg) after 8 weeks of supplementation, the difference and rate of increase in other NR metabolites levels in urine between placebo and NIAGEN (100 mg, 300 mg, and 1000 mg) after 8 weeks of supplementation, and the difference in other NR metabolites levels in muscle between placebo and NIAGEN (100 mg, 300 mg, and 1000 mg) after 8 weeks of supplementation. Exploratory outcomes included exploring the changes in Resting Energy Expenditure (REE) relative to placebo after 8 weeks of supplementation, the changes in blood levels of branched-chain amino acids relative to placebo after 8 weeks of supplementation, the changes in blood levels of high sensitivity C-reactive protein (hsCRP) relative to placebo after 8 weeks of supplementation. The safety objectives included the difference in vital signs, hematology and clinical chemistry parameters including high density lipoprotein cholesterol (HDL-C), LDL-C, triglycerides, and total cholesterol between the placebo- and NIAGEN-treated groups, and the difference in the incidence of adverse events between the placebo- and NIAGEN-treated groups. The effect of NIAGEN on plasma HCY levels was determined as a post hoc analysis. There were no changes to the trial outcomes or method during the trial and interim analyses were not conducted.
Healthy men and non-pregnant, non-breastfeeding women (40–60 years of age) were eligible for the study if their body mass index was between 25–30, they were willing to avoid vitamin B3 supplements and limit ingestion of foods containing moderate amounts of tryptophan and vitamin B3, maintain current levels of physical activity throughout the study, and refrain from caffeine consumption on days when study visits included blood collection for metabolite measurement. Women of childbearing potential were eligible only if willing to use medically approved forms of birth control. Individuals with diabetes, active peptic ulcer disease, alcohol use >2 standard servings/day or history of drug or alcohol abuse in the past year, using medical marijuana, anti-hypertensives, or lipid lowering medications were excluded. Individuals with a history of renal disease, liver disease, or history of niacin deficiency were also excluded. Individuals were determined healthy by laboratory results, medical exam and physical exam. Informed consent was obtained from each participant at the screening prior to any study-related activities being performed.
Randomization
The participants were assigned to the different groups by simple randomization. Participants were identified by their initials and their date of birth and were assigned a participant number at their screening visit. If the potential participant met all the inclusion criteria and did not meet any of the exclusion criteria at baseline, a randomization number was assigned to the participant by a blinded investigator per the order of the randomization list generated by www.randomization.com .
Study product
The study consisted of a 2-week run-in and 8-week interventional period. Participants received either 100 mg, 300 mg, 1000 mg NR per day or placebo during the 8-week intervention. The NR capsule consisted of 100 mg or 250 mg of NR chloride (99% purity) as the active ingredient and microcrystalline cellulose and vegetarian capsule as non-active ingredients. The placebo capsule consisted of microcrystalline cellulose and a vegetarian capsule. No differences in size, color, taste, texture, or packaging were detectable between the two products. The investigational products and the placebo capsules were sealed in identically-appearing blister packets, which were labelled per ICH-GCP and applicable local regulatory guidelines. Unblinded personnel at KGK Science Inc., who were not involved in any study assessments, labelled the investigational product. A randomization schedule was created and provided to the investigator indicating the order of randomization. Investigators, other site personnel, and participants were blinded to the product.
Participants were instructed to take 4 capsules daily after breakfast beginning the day after their randomization visit (Day 1). The 4 capsules amounted to a single dose of either placebo (a total of 4 placebo capsules) or 100 mg NR (1 capsule containing 100 mg NR and three placebo capsules), 300 mg NR (3 capsules containing 100 mg NR and 1 capsule containing placebo) or 1000 mg NR (4 capsules containing 250 mg NR). Participants were instructed to save all unused and open packages and return them at each visit for a determination of compliance. Compliance to the protocol was also assessed by reviewing the 3-day food record and study diaries completed by each participant for adherence to the study’s dietary restrictions, ingestion of the investigational product, and maintenance of physical activity levels.
Laboratory measurements
Subjects fasted for 12 hours prior to study visits.
Anthropometric measures and vitals were assessed at screening, day 0, 7, 14, 28 and 56. Blood was collected for the assessment of laboratory parameters (CBC, electrolytes Na, K, Cl, HbA1c, creatinine, BUN, AST, ALT, GGT, and bilirubin) at screening, day 0, 7, 14, 28 and 56, blood lipids and NAD + metabolite analyses on day 0, 7, 14, 28, and 56. Urine was also collected for NAD + metabolites analyses on day 0, 7, 14, 28, and 56. The assessments of laboratory parameters and blood lipids were conducted by LifeLabs (Etobicoke, Ontario, Canada) using standardized procedures. NAD + metabolites in blood and urine were quantitated by LC-MS-MS at Keystone Bioanalytical, Inc. (North Wales, PA) using analytically validated methods in accordance with Good Laboratory Practices. Only metabolite data from participants who completed the study and had metabolite levels above the limit of quantitation were included in the analysis.
For whole blood NAD + analysis, NAD + - 13 C 5 was the internal standard. The lower limit of quantification (LLOQ) was 0.3 µg/ml, the upper limit of the quantification (ULOQ) was 50 µg/ml, and the inter-assay precision (% CV) was 1.10 to 11.83%. Plasma NAM was quantified against a NAM-d4 standard with a LLOQ = 5 ng/ml, ULOQ = 3000 ng/ml, and a % CV of 0.71 to 5.38%. Plasma MeNAM was quantified against an MeNAM-d3 standard with a LLOQ = 4 ng/ml, ULOQ = 2000 ng/ml, and a %CV of 0.34 to 13.31%. Urinary MeNAM and N 1 -methyl-2-pyridone-5-carboximide (Me2PY) were quantified against internal d3 standards with an LLOQ = 1 µg/ml and ULOQ = 256 µg/ml for both analytes. The %CV for urinary MeNAM and Me2PY were 1.25 to 4.60% and 1.10 to 3.22%, respectively.
Plasma HCY levels were quantified by LC-MS-MS at Keystone Bioanalytical. Sodium citrate-treated plasma was pretreated with 50 µL of 0.5 M DTT (1,4-dithiothreitol) and HCY and the internal standard (HCY-d4) were precipitated using 0.5% formic acid and 0.05% TFA in acetonitrile. After vortexing and centrifuging, 20 µL of the supernatant was diluted in 200 µL of nano-pure water in a clean HPLC vial, and 5–10 µL was injected into the liquid chromatography mass spectrometer. The standard curve range was 0.2–40 µg/mL with the LLOQ of 0.2 µg/mL.
Adverse events (AEs)
Subjects were instructed to record any AEs in a diary and were asked at each visit if they have experienced any difficulties or problems since the last visit.
Statistical analyses
Statistical analyses were completed using the R Statistical Software Package Version 3.2.1 (R Core Team, 2015) for Microsoft Windows. All statistical analyses were performed at a significance level of 5%. Although the primary outcome variable was the difference in urinary MeNAM levels between placebo and NR (100 mg, 300 mg, 1000 mg) treated subjects after 8 weeks of supplementation, the study was powered for a secondary outcome of elevation of blood NAD + . Statistical power was based on the estimated standard deviation of 10.1 µM for blood NAD + levels 46 and 80% power to detect an effect size of at least an 8.7 µM increase. With attrition estimated at 20% throughout the course of the study, a total of 140 subjects were enrolled. For reference, if the study had been powered to detect a significant increase in MeNAM levels, then a total of 128 subjects would have been required.
Statistical analyses were performed on a modified intent-to-treat population (ITT), which consisted of all subjects who received either product, and on whom any post-randomization efficacy information is available. Variables were tested for normality and log-normality where log-normality distributed variables were analyzed in the logarithmic domain. Appropriate non-parametric tests were used to analyze non-normal variables. All missing values were imputed with last observation carried forward (LCOF) imputation. No imputation was performed for missing values of safety variables.
Numerical endpoints were formally tested for significance between groups by analysis of covariance (ANCOVA). The dependent variable was the value at each visit, the factor was the treatment group, and the value at baseline (Day 0) was the covariate. When the effect of supplementation was significant (p-value ≤ 0.05), the pairwise Tukey-Kramer post hoc test was applied. Significant efficacy of the product, relative to placebo, was inferred if the coefficient of the treatment group in the ANCOVA model was significantly different from zero (p ≤ 0.05). Numerical endpoints that are intractably non-normal were assessed by the Mann-Whitney U test. A within group analysis on efficacy endpoints was done using the Student’s paired t-test or, in the case of intractable non-normality, the Wilcoxon sign rank test was performed.
Compliance and completion of the clinical study
Two hundred and eighty-six subjects were screened against the eligibility requirements. One hundred and forty subjects with an age range of 40–60 years and a body mass index of 25.0–30.1 kg/m 2 were deemed healthy per their screening laboratory values (complete blood panel, hematology, electrolytes, and liver and kidney function tests) and enrolled in the study (Fig. 2 ). After the 2-week run-in period, subjects were randomized to one of four treatment groups (placebo; 100 mg NR/day; 300 mg NR/day; 1000 mg NR/day). There were no significant differences in any of the screening laboratory values between the different groups. There were also no differences in demographics, anthropometric measurements or vital signs between groups (Table 1 ). The first potential participant was screened on March 1, 2016 and the last participant’s last visit was on March 17, 2017. The trial was ended after the last randomized subject completed the last visit.
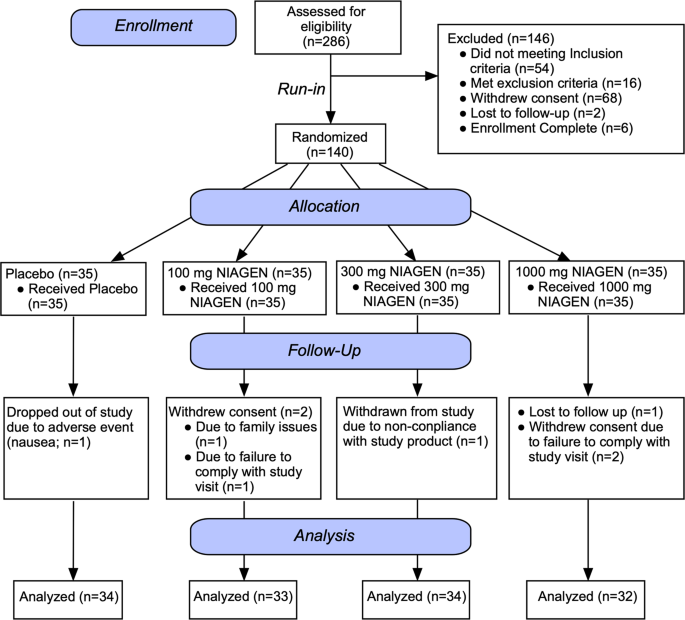
Disposition of the study participants. Two hundred and eighty-six men and women were screened for eligibility. One hundred and forty subjects met the eligibility criteria and were enrolled in the study. After the 2-week run-in (Day 0), the subjects were randomized to one of four treatment groups (Placebo, 100 mg, 300 mg, or 1000 mg NIAGEN per day; n = 35/group). Over the course of the 56-day supplementation period, one subject withdrew from the placebo-treated group due to an adverse event, two subjects withdrew consent in the 100 mg NIAGEN treated group, one subject was withdrawn from the 300 mg NIAGEN-treated group and two subjects withdrew consent and one was lost to follow-up in the 1000 mg NIAGEN-treated group.
Seven participants failed to complete the study (Fig. 2 ). One subject dropped out of the placebo group due to nausea, one subject was withdrawn from the 300 mg NR-treated group due to non-compliance with the study product, four subjects in the 100 and 1000 mg NR-treated groups withdrew consent (100 mg NR, n = 2; 1000 mg NR n = 2), and one subject in the 1000 mg NR group was lost to follow-up.
Compliance to NR, measured by counting unused capsules returned to the study site, was 98% with a mean compliance of 97.5% in the 100 mg/day NR group, 98.6% in the 300 mg/d group, 97.1% in the 1000 mg/d group, and 99% for participants in the placebo group. Based on dietary records maintained by the subjects, there were no significant between-group differences in total caloric intake or intake of forms of vitamin B3 during the course of the trial.
NR produces dose-dependent increases in blood and urinary NAD + metabolites
Blood nad +.
NAD + levels in peripheral blood mononuclear cells (PBMCs) peak 8 hours after the administration 300 and 1000 mg of NR 20 . However, the time course and dose-dependency by which oral NR increases steady-state NAD + levels in whole blood is not known. Relative to baseline, small but significant decreases in blood NAD + levels occurred in the placebo group over the 56-day supplementation period (p < 0.05). In contrast, daily doses of 300 mg and 1000 mg NIAGEN significantly (p < 0.05) increased NAD + within seven days relative to baseline and placebo (Fig. 3A ) and were sustained for the remainder of the study. Blood NAD + levels in the 100 mg-treated group were significantly increased at day 14 relative to baseline and similar to the placebo group at all time points. The day 56 whole blood NAD + level and the rate of change effect sizes also increased dose-dependently to 1.74 and 1.98, respectively (Supplemental Tables 3 and 4 ). At day 14, the blood NAD + levels of the 100 mg, 300 mg and 1000 mg participants were increased by 22 ± 9%, 51 ± 7% and 142 ± 14% with respect to their baseline blood NAD + levels. At day 56, the blood NAD + levels of the same 100 mg, 300 mg and 1000 mg participants were sustained at increases of 10% ± 4%, 48 ± 8% and 139 ± 19% with respect to their baseline blood NAD + levels.
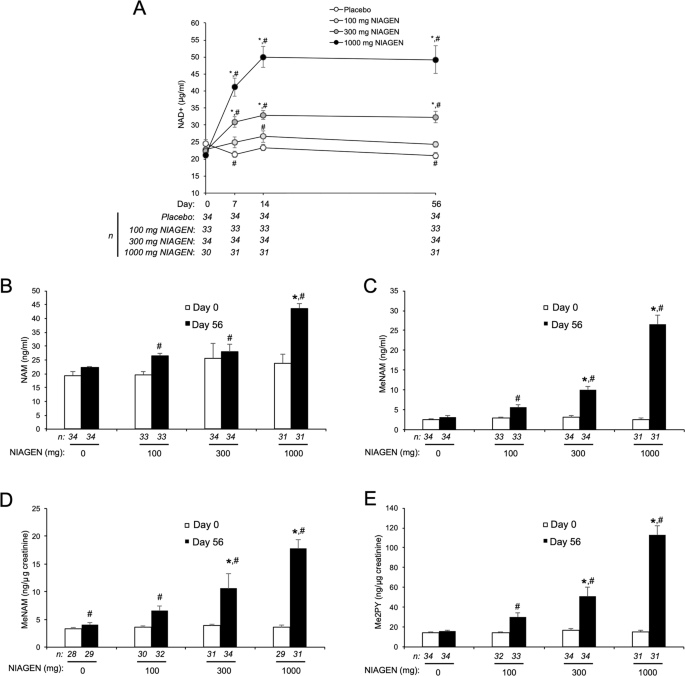
NIAGEN supplementation significantly increases NAD + and other NAD + metabolites. ( A ) Whole blood levels of NAD + in the intent-to-treat (ITT) population over the course of 56 days of placebo, 100, 300, or 1000 mg of NIAGEN per day supplementation. ( B ) Plasma nicotinamide (NAM); ( C ) Plasma 1-methylnicotinamide MeNAM; ( D ) urinary (MeNAM); and ( E ) urinary N-methyl-2-pyridone-3/5-carboximide (Me2PY) levels in the ITT population before and after 56 days of supplementation with placebo, 100, 300, or 1000 mg of NIAGEN per day. Urinary MeNAM and Me2PY levels were normalized to urinary creatinine concentrations. Asterisks denote significant (p < 0.05) between group differences versus placebo. Number signs denote significant (p < 0.05) within group differences relative to Day 0. Error bars represent standard error of the mean. Only data from participants who completed the study and had metabolite levels above the limit of quantitation were included in the analysis. Data for within group differences in panels A, B, C and E were transformed logarithmically to achieve normality.
Plasma and urinary metabolites
NAD + -consuming enzymes such as the sirtuins, PARP, and cyclic ADPribose synthases hydrolyze the linkage between the NAM and the ADPribosyl moieties of NAD + , producing NAM and ADPribosyl products 14 , 47 , 48 . NAM then circulates and is methylated in the liver and other tissues to MeNAM 49 , 50 , 51 . Both plasma and urinary blood MeNAM and its oxidation products Me2PY and Me4PY are considered to be biomarkers of increased NAD + metabolism 52 . Fifty-six days of supplementation with NR resulted a significant (p < 0.05) increase in plasma NAM in the 1000 mg group compared to placebo (Fig. 3B ) with an effect size of 1.21 (Supplemental Table 5 ). Relative to baseline, significant (p < 0.05) increases in plasma NAM were also detected in the 100, 300 and 1000 mg-treated groups. Correspondingly, plasma and urinary levels of MeNAM and Me2PY were also significantly (p < 0.05) and dose-dependently increased in the 300 and 1000 mg-treated groups compared to placebo (Fig. 3C–E ), resulting in day 56 metabolite level and rate of change effect sizes that ranged from 0.49 to 2.85 and increased with the amount of NIAGEN ingested (Supplemental Tables 1 , 2 , 7 , 8 , 9 and 10 ). Significant (p < 0.05) and dose-dependent increases plasma and urinary levels of MeNAM and Me2PY relative with baseline were also noted in the 100, 300 and 1000 mg groups (Fig. 3C–E ).
Oral NIAGEN is safe and well-tolerated up to 1000 mg/day for 8 weeks
No dose-dependent aes.
AEs were coded with Medical Dictionary for Regulatory Activities version 17.0. According to this coding system, flushing (flushing, feeling of warmth transient, hot flush) would be reported under the general disorders and administration site conditions. Ninety-five AEs were reported by 61 participants (Table 2 ). There were no serious AEs or reports of flushing. Moreover, the type, incidence and severity of the AEs were similar across the different groups.
Of the 26 AEs reported in the 100 mg NR group, 24 were reported as being unlikely or not related to the study product. The 2 AEs reported as being possibly related were leg pain and high blood pressure and were mild in intensity. Of the 27 AEs reported in the 300 mg NR group, 25 were reported as being unlikely or not related to the study product. The 2 AEs reported as being possibly related were nausea and muscle pain and were mild in intensity. Of the 22 AE reported in the 1000 mg NR group, 19 were reported as being unlikely or not related to the study product. The 3 AEs reported as being possibly related were sore back, muscle soreness and nausea and were all mild in intensity. Of the 20 AEs reported in the placebo group, 16 were reported as being unlikely to the study product. Of the 4 AEs reported as being possible related, 3 were mild in intensity (rash, raised liver function tests, nausea) and 1 was moderate in intensity (upset stomach). Importantly, all AEs were resolved by the end-of-study.
Vital signs
There were no between-group differences in mean systolic blood pressure, mean diastolic blood pressure, mean heart rate or weight. Further, all within-group changes were within normal clinical ranges and were not of clinical significance for this population.
Hematology and clinical chemistry
Some differences were observed in the hematology parameters at day 56 (Table 3 , Supplemental Figure). Specifically, decreases occurred in the white blood cell count and monocyte count in the placebo-treated group, white blood cell, neutrophil, and lymphocyte counts in the 100 mg-treated group, white blood cell, neutrophil, lymphocyte, monocyte, and basophil counts in the 300 mg-treated group, and the white blood cell, neutrophil, and lymphocyte counts in the 1000 mg-treated group. In contrast, increases in mean corpuscular volume, mean corpuscular hemoglobin, and red cell distribution width occurred only in the 1000 mg-treated group. Statistically significant differences also occurred in the white blood cell count in the 300 mg group compared to the placebo-, 100 mg-, and 1000 mg-treated groups and the red cell distribution width in 1000 mg-treated group compared to placebo-, 100 mg-, and 300 mg-treated groups. Importantly, the differences were not dose-dependent, within the healthy clinical reference ranges for the laboratory and clinic location, and deemed to be not clinically meaningful or an AE.
Recently, dose-dependent, statistically significant increases in total cholesterol and LDL-C were observed in a clinical study in which participants received a combination of 250 mg NR plus 50 mg pterostilbene or a combination of 500 mg NR plus 100 mg pterostilbene for eight weeks 42 . As shown in Table 4 , there were no statistically significant differences in the NIAGEN and placebo-groups with respect to any clinical chemistry parameter. Clinical testing of pterostilbene alone indicates that it produces time and dose-dependent increases in human LDL-C 53 of a magnitude that are a public health concern 54 , 55 and are inconsistent with pterostilbene being a sirtuin 1 activator or included as part of a consumer wellness product 56 .
NR and plasma homocysteine
Nicotinamide N-methyltransferase catalyzes the transfer of a methyl group from S -adenosylmethionine (SAM) to NAM, generating to MeNAM and S -adenosylhomocysteine 49 , 50 , 51 . S -adenosylhomocysteine is then subsequently cleaved to homocysteine (HCY) and adenosine. It has been reported that single 300 mg oral doses of NA and NAM increase plasma HCY levels 23 , indicating a potential shortage of methyl groups that could be needed for formation of molecules such as dopamine and creatine. Moreover, increased plasma HCY is an independent risk factor for the development of vascular disease 57 , 58 , 59 . To determine whether prolonged ingestion of NR increases plasma HCY levels, a post hoc analysis was conducted using sodium citrate-treated plasma samples collected during the study. Compared to baseline or the placebo-treated group, NR ingestion had no effect on plasma HCY levels (Fig. 4 ).
NIAGEN supplementation does not disturb plasma homocysteine. Plasma HCY levels in the intent-to-treat population before and after 56 days of supplementation with placebo, 100, 300, or 1000 mg of NIAGEN per day. Error bars represent standard error of the mean. Only data from participants who completed the study and had metabolite levels above the limit of quantitation were included in the analysis.
Exploratory endpoints
No significant differences between the any of the NR- and placebo-treated groups were seen in either the REE, blood levels of branched-chain amino acids, or hsCRP after 8 weeks of supplementation.
Because NAD + is the most abundant NAD + metabolite in any cellular sample 60 , it is the breakdown of NAD + and NAD + -related coenzymes in food that produces the three salvageable NAD + precursor vitamins: NR, NAM and NA. In addition to the existence of NR in milk 5 , 61 and apart from the availability of NR as a supplement, mammals are exposed to NR from the digestive breakdown of dietary NAD + and endogenous NR circulation. Endogenous NR has been shown to be a critical nutrient in maintaining health as mice lacking the major NR kinase gene have depressed hepatic NAD + and depressed liver function 62 . In addition, people undergoing heart failure increase their cardiac expression of the NR kinase 2 gene 10 . This only makes sense if NR is an endogenous form of B3.
NR has been demonstrated to be safe and GRAS, supported by a rigorous battery of animal toxicology studies 37 . Additionally, NR was well-tolerated in all published clinical studies 20 , 41 , 43 , 44 . Because NA use is limited by flushing, it was of particular interest to assess whether there would be reports of flushing or other treatment related AEs that are associated with ingestion of NR. Here we show in a randomized, placebo-controlled, double-blind, parallel-group study involving 140 overweight, otherwise healthy adults that the ingestion of up 1000 mg of NR is not associated with flushing. Limitations of the study were that it was conducted in predominantly white, middle-aged adults who consumed a diet limited in niacin equivalents.
The concept of niacin equivalence among the NAD + precursors is clearly useful when defining reference intakes because adequate amounts of tryptophan, NAM or NA can prevent pellagra 7 . However, niacin equivalency does not apply at the higher doses used to support other health endpoints as evidenced by the independent ULs for NAM and NA derived by the European Commission and UK Expert Group on Vitamins and Minerals. The UL for NA was established at 10 mg/day based on flushing 63 and the UL for NAM is 900 mg/day based on the NOAELs established in clinical studies administering doses up to 3 g NAM per day 64 . Additionally, on the basis of elevating HCY, a sensitive biomarker of methylation status, NAM and NA differ in terms of their potential to dysregulate 1-carbon metabolism. While both of the classical forms of B3 elevated plasma HCY after single doses of 300 mg, NAM elevated HCY substantially more than NA 23 . On a molar basis, 300 mg of NAM (MW = 122 Da) is equivalent to 716 mg of NR Cl (MW = 291 Da) and our data show that NR does not elevate HCY at daily doses up to 1000 mg for 8 weeks.
NR, NAM and NA are converted to NAD + through three different gene-encoded pathways that are tissue-restricted in the case of NA 2 . Because NA uniquely produces flushing, there is a reason for a lower UL for NA. Additionally, although NAM does not appear to produce AEs, there is some concern around its use as a vitamin due to its ability to dysregulate 1-carbon metabolism 23 and inhibit sirtuins at high doses 20 , 22 . The safe oral availability of NR and its lack of adverse effects on HCY and LDL-C at doses up to 1000 mg/day support the establishment of a UL for NR that is equal to or greater than that of NAM.
Data Availability
The datasets generated during and/or analyzed during the current study are available by request.
Belenky, P., Bogan, K. L. & Brenner, C. NAD+metabolism in health and disease. Trends in Biochemical Sciences 32 , 12–19, https://doi.org/10.1016/j.tibs.2006.11.006 (2007).
Article CAS PubMed Google Scholar
Bogan, K. L. & Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+precursor vitamins in human nutrition. Annual Review of Nutrition 28 , 115–130, https://doi.org/10.1146/annurev.nutr.28.061807.155443 (2008).
Verdin, E. NAD + in aging, metabolism, and neurodegeneration. Science 350 , 1208–1213 (2105).
Article Google Scholar
Mouchiroud, L. et al . The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 154 , 430–441, https://doi.org/10.1016/j.cell.2013.06.016 (2013).
Article CAS PubMed PubMed Central Google Scholar
Bieganowski, P. & Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+in fungi and humans. Cell 117 , 495–502, https://doi.org/10.1016/S0092-8674(04)00416-7 (2004).
IOM. Dietary Reference Intakes for Thiamine, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. (National Academy Press, 1998).
Horwitt, M. K. Niacin-tryptophan relationships in the development of pellagra. The American journal of clinical nutrition 3 , 244–245 (1955).
Article CAS Google Scholar
Trammell, S. A. et al . Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Scientific reports 6 , 26933, https://doi.org/10.1038/srep26933 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Brown, K. D. et al . Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 20 , 1059–1068 (2014).
Diguet, N. et al . Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 137 , 2256–2273, https://doi.org/10.1161/CIRCULATIONAHA.116.026099 (2018).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. Neurobiology. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348 , 453–457, https://doi.org/10.1126/science.1258366 (2015).
Vaur, P. et al . Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J 31 , 5440–5452, https://doi.org/10.1096/fj.201700221RR (2017).
Ear, P. H. et al . Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring. Cell Rep 26 , 969–983 e964, https://doi.org/10.1016/j.celrep.2019.01.007 (2019).
Bouchard, V. J., Rouleau, M. & Poirier, G. G. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol 31 , 446–454 (2003).
Zakhari, S. & Li, T. K. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology 46 , 2032–2039, https://doi.org/10.1002/hep.22010 (2007).
Braidy, N. et al . Mapping NAD(+) metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology 15 , 177–198, https://doi.org/10.1007/s10522-013-9489-5 (2014).
Massudi, H., Grant, R., Guillemin, G. J. & Braidy, N. NAD + metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox report: communications in free radical research 17 , 28–46, https://doi.org/10.1179/1351000212y.0000000001 (2012).
Zhou, C. C. et al . Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. British journal of pharmacology 173 , 2352–2368, https://doi.org/10.1111/bph.13513 (2016).
Imai, S. & Guarente, L. NAD + and sirtuins in aging and disease. Trends in cell biology 24 , 464–471, https://doi.org/10.1016/j.tcb.2014.04.002 (2014).
Trammell, S. A. et al . Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7 , 12948, https://doi.org/10.1038/ncomms12948 (2016).
Guyton, J. R. & Bays, H. E. Safety considerations with niacin therapy. The American journal of cardiology 99 , 22c–31c, https://doi.org/10.1016/j.amjcard.2006.11.018 (2007).
Bitterman, K. J., Anderson, R. M., Cohen, H. Y., Latorre-Esteves, M. & Sinclair, D. A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1. Journal of Biological Chemistry 277 , 45099–45107 (2002).
Sun, W. P. et al . Comparison of the effects of nicotinic acid and nicotinamide degradation on plasma betaine and choline levels. Clin Nutr 36 , 1136–1142, https://doi.org/10.1016/j.clnu.2016.07.016 (2017).
Belenky, P. et al . Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD + . Cell 129 , 473–484, https://doi.org/10.1016/j.cell.2007.03.024 (2007).
Canto, C. et al . The NAD(+) Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab 15 , 838–847, https://doi.org/10.1016/j.cmet.2012.04.022 (2012).
Vannini, N. et al . The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell Stem Cell 24 , 405–418 e407, https://doi.org/10.1016/j.stem.2019.02.012 (2019).
Hamity, M. V. et al . Nicotinamide riboside, a form of vitamin B3 and NAD+precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain 158 , 962–972, https://doi.org/10.1097/j.pain.0000000000000862 (2017).
Sasaki, Y., Araki, T. & Milbrandt, J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci 26 , 8484–8491 (2006).
Yoshino, J., Baur, J. A. & Imai, S. I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab 27 , 513–528, https://doi.org/10.1016/j.cmet.2017.11.002 (2018).
Grozio, A. et al . Slc12a8 is a nicotinamide mononucleotide transporter. Nature Metabolism 1 , 47–57 (2019).
Schmidt, M. S. & Brenner, C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nature Metabolism in press (2019).
Ratajczak, J. et al . NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun 7 , 13103, https://doi.org/10.1038/ncomms13103 (2016).
Nikiforov, A., Dolle, C., Niere, M. & Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem 286 , 21767–21778, https://doi.org/10.1074/jbc.M110.213298 (2011).
Grozio, A. et al . CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem 288 , 25938–25949, https://doi.org/10.1074/jbc.M113.470435 (2013).
Sociali, G. et al . Antitumor effect of combined NAMPT and CD73 inhibition in an ovarian cancer model. Oncotarget 7 , 2968–2984, https://doi.org/10.18632/oncotarget.6502 (2016).
Article PubMed Google Scholar
Fletcher, R. S. et al . Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Molecular metabolism 6 , 819–832, https://doi.org/10.1016/j.molmet.2017.05.011 (2017).
Conze, D. B., Crespo-Barreto, J. & Kruger, C. L. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol . https://doi.org/10.1177/0960327115626254 (2016).
FDA. GRAS Notification for Nicotinamide riboside chloride (2016).
FDA. New Dietary Ingredient Notification 882 for Nicotinamide riboside chloride (2015).
FDA. New Dietary Ingredient Notification 1062 for Nicotinamide riboside chloride (2018).
Airhart, S. E. et al . An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD + levels in healthy volunteers. PLoS One 12 , e0186459, https://doi.org/10.1371/journal.pone.0186459 (2017).
Dellinger, R. W. et al . Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis 3 , 17, https://doi.org/10.1038/s41514-017-0016-9 (2017).
Martens, C. R. et al . Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun 9 , 1286, https://doi.org/10.1038/s41467-018-03421-7 (2018).
Dollerup, O. L. et al . A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. The American journal of clinical nutrition 108 , 343–353, https://doi.org/10.1093/ajcn/nqy132 (2018).
Dolopikou, C. F. et al . Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. European journal of nutrition , https://doi.org/10.1007/s00394-019-01919-4 (2019).
Fu, C. S., Swendseid, M. E., Jacob, R. A. & McKee, R. W. Biochemical markers for assessment of niacin status in young men: levels of erythrocyte niacin coenzymes and plasma tryptophan. The Journal of nutrition 119 , 1949–1955, https://doi.org/10.1093/jn/119.12.1949 (1989).
Finkel, T., Deng, C. X. & Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 460 , 587–591, https://doi.org/10.1038/nature08197 (2009).
Okamoto, H., Takawasa, S. & Sugawara, A. The CD38-Cyclic ADP-Ribose System in Mammals: Historical Background, Pathophysiology and Perspective. Messenger 3 , 27–24 (2014).
Swiatek, K. R., Simon, L. N. & Chao, K. L. Nicotinamide methyltransferase and S-adenosylmethionine: 5’-methylthioadenosine hydrolase. Control of transfer ribonucleic acid methylation. Biochemistry 12 , 4670–4674 (1973).
Cantoni, G. L. Methylation of nicotinamide with soluble enzyme system from rat liver. J Biol Chem 189 , 203–216 (1951).
CAS PubMed Google Scholar
Trammell, S. A. & Brenner, C. NNMT: A Bad Actor in Fat Makes Good in Liver. Cell Metab 22 , 200–201, https://doi.org/10.1016/j.cmet.2015.07.017 (2015).
Mrochek, J. E., Jolley, R. L., Young, D. S. & Turner, W. J. Metabolic response of humans to ingestion of nicotinic acid and nicotinamide. Clin Chem 22 , 1821–1827 (1976).
Riche, D. M. et al . Pterostilbene on metabolic parameters: a randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med 2014 , 459165, https://doi.org/10.1155/2014/459165 (2014).
Article PubMed PubMed Central Google Scholar
Goff, D. C. et al . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 63 , 2935–2959, https://doi.org/10.1016/j.jacc.2013.11.005 (2014).
Stone, N. J. et al . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 63 , 2889–2934, https://doi.org/10.1016/j.jacc.2013.11.002 (2014).
Brenner, C. & Boileau, A. C. Pterostilbene raises low density lipoprotein cholesterol in people. Clin Nutr 38 , 480–481, https://doi.org/10.1016/j.clnu.2018.10.007 (2019).
Boushey, C. J., Beresford, S. A., Omenn, G. S. & Motulsky, A. G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. Jama 274 , 1049–1057 (1995).
Garg, R., Malinow, M., Pettinger, M., Upson, B. & Hunninghake, D. Niacin treatment increases plasma homocyst(e)ine levels. American heart journal 138 , 1082–1087 (1999).
Basu, T. K., Makhani, N. & Sedgwick, G. Niacin (nicotinic acid) in non-physiological doses causes hyperhomocysteineaemia in Sprague-Dawley rats. The British journal of nutrition 87 , 115–119, https://doi.org/10.1079/bjn2001486 (2002).
Trammell, S. A. & Brenner, C. Targeted, LCMS-based Metabolomics for Quantitative Measurement of NAD(+) Metabolites. Comput Struct Biotechnol J 4 , e201301012, https://doi.org/10.5936/csbj.201301012 (2013).
Trammell, S. A., Yu, L., Redpath, P., Migaud, M. E. & Brenner, C. Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk. The Journal of nutrition 146 , 957–963, https://doi.org/10.3945/jn.116.230078 (2016).
Fan, R. et al . Overexpression of NRK1 ameliorates diet- and age-induced hepatic steatosis and insulin resistance. Biochemical and biophysical research communications 500 , 476–483, https://doi.org/10.1016/j.bbrc.2018.04.107 (2018).
Sebrell, W. H. B. R. E. A reaction ot the oral administration of nicotinic acid. JAMA 1111 , 2286–2287 (1938).
Scientific Committee on Food. Opinion of the Scientific Committee on Food on the tolerable upper intake level of nicotinic acid and nicotinamide (niacin) (2002).
Download references
Acknowledgements
The authors thank Allan Xu of Keystone Bioanalytical for quantifying plasma HCY levels, and the analytical method validation and sample analysis for NAD + and related metabolites in blood, plasma and urine.
Author information
Dietrich Conze and Claire L. Kruger contributed equally.
Authors and Affiliations
Chromadex Spherix Consulting, 11821 Parklawn Drive, Suite 310, Rockville, MD, 20852, United States
Dietrich Conze & Claire L. Kruger
Department of Biochemistry, University of Iowa, 4-403 BSB, Iowa City, IA, 52242, United States
Charles Brenner
You can also search for this author in PubMed Google Scholar
Contributions
D.C., C.B. and C.K. designed the study. All authors analyzed data, wrote and approved the manuscript.
Corresponding authors
Correspondence to Charles Brenner or Claire L. Kruger .
Ethics declarations
Competing interests.
Claire Kruger and Dietrich Conze are employees of ChromaDex. Charles Brenner is the inventor of intellectual property licensed by ChromaDex and serves as their chief scientific adviser. ChromaDex funded the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Final protocol, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Conze, D., Brenner, C. & Kruger, C.L. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci Rep 9 , 9772 (2019). https://doi.org/10.1038/s41598-019-46120-z
Download citation
Received : 26 March 2019
Accepted : 21 June 2019
Published : 05 July 2019
DOI : https://doi.org/10.1038/s41598-019-46120-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impact of nad+ metabolism on ovarian aging.
- Jinghui Liang
- Feiling Huang
Immunity & Ageing (2023)
Mitochondrial dysfunction in Parkinson’s disease – a key disease hallmark with therapeutic potential
- Martin T. Henrich
- Wolfgang H. Oertel
- Fanni F. Geibl
Molecular Neurodegeneration (2023)
NR-SAFE: a randomized, double-blind safety trial of high dose nicotinamide riboside in Parkinson’s disease
- Haakon Berven
- Simon Kverneng
- Charalampos Tzoulis
Nature Communications (2023)
NAD+ Precursors Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR): Potential Dietary Contribution to Health
- Gabriela Fabiana Soares Alegre
- Glaucia Maria Pastore
Current Nutrition Reports (2023)
Importance of NAD+ Anabolism in Metabolic, Cardiovascular and Neurodegenerative Disorders
- Tessa Helman
- Nady Braidy
Drugs & Aging (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

IMAGES
VIDEO
COMMENTS
Nicotinamide riboside (NR) has recently become one of the most studied nicotinamide adenine dinucleotide (NAD +) precursors, due to its numerous potential health benefits mediated via elevated NAD + content in the body. NAD + is an essential coenzyme that plays important roles in various metabolic pathways and increasing its overall content has been confirmed as a valuable strategy for ...
All the research efforts have also resulted in the development of dietary recommendations and vitamin supplementation for ... A reduced form of nicotinamide riboside defines a new path for NAD(+) biosynthesis and acts as an orally bioavailable NAD(+) precursor. Mol. Metab. 2019; 30:192-202. doi: 10.1016/j.molmet.2019.09.013. [PMC free ...
Nicotinamide riboside is a precursor to the important cofactor nicotinamide adenine dinucleotide and has elicited metabolic benefits in multiple preclinical studies. ... This review endeavors to summarize and critically assess the 25 currently published research articles on human nicotinamide riboside supplementation to identify any poorly ...
Oral nicotinamide riboside (NR) at a dose of 3000 mg daily for 30 days is safe and associated with a pronounced systemic augmentation of the NAD metabolome, but no methyl donor depletion.
Nicotinamide riboside and doubly labeled nicotinamide riboside (13C1, H 2-1) were obtained from ChromaDex Inc. (Irvine, CA). All HPLC solvents and extraction solvents were HPLC grade or better.
1. Introduction. Nicotinamide riboside (NR) is widely used as a dietary supplement. Structurally, it is a form of vitamin B 3 (nicotinic acid, niacin, NA), incorporating into its structure more elements of nicotinamide adenine dinucleotide (in its oxidized form, NAD +) [].NR influences, in particular, energy metabolism and neuroprotection [2,3,4].From a non-medical perspective, as a part of ...
Here, the authors show nicotinamide riboside treatment shrinks the age-enlarged stem cell pool and shifts aged HSC functionally, metabolically and molecularly towards the young state.
Nicotinamide riboside (NR) has recently become one of the most studied nicotinamide adenine dinucleotide (NAD+) precursors, due to its numerous potential health benefits mediated via elevated NAD+ content in the body. NAD+ is an essential coenzyme that plays important roles in various metabolic pathways and increasing its overall content has been confirmed as a valuable strategy for treating a ...
A bioluminescent-based probe for in vivo non-invasive monitoring of nicotinamide riboside uptake reveals a link between metastasis and NAD+ metabolism. Biosensors and Bioelectronics , 2023; 220: ...
Researchers have determined that the naturally occurring dietary supplement, nicotinamide riboside (NR), can enter the brain. The finding is significant because it supports the idea that NR, upon ...
A type of vitamin B3, called nicotinamide riboside, alleviates persistent pain in mice, suggesting that it may potentially treat chronic pain in humans as well. Inflammation - the body's first ...
Summary: Nicotinamide riboside (NR), a naturally occurring dietary supplement can enter the brain and alter the metabolism of biological pathways associated with Alzheimer's disease. Source: University of Delaware For the first time, a researcher at the University of Delaware College of Health Sciences in collaboration with a team at the National Institute on Aging, a division of the ...
The clinical trial was part of the ChromaDex External Research Program (CERP™), which donated ChromaDex's patented nicotinamide riboside (NR) ingredient, Niagen ®, one of the most efficient ...
At 12 months, the rate of new nonmelanoma skin cancers was lower by 23% (95% confidence interval [CI], 4 to 38) in the nicotinamide group than in the placebo group (P=0.02).
Nicotinamide riboside (NR) is an NAD+ precursor capable of regulating mammalian cellular metabolism. ... the animals were fed an experimental 60% HFD (D12492, Research Diets, Inc.) or a matched ...
Among all the NAD+ precursors, nicotinamide riboside (NR) has gained the most attention as a potent NAD+-enhancement agent. This recently discovered vitamin, B3, has demonstrated excellent safety and efficacy profiles and is orally bioavailable in humans. Boosting intracellular NAD+ concentrations using NR has been shown to provide protective effects against a broad spectrum of pathological ...
While previous studies have linked commercial dietary supplements like nicotinamide riboside (NR), a form of vitamin B3, to benefits related to cardiovascular, metabolic and neurological health, new research from the University of Missouri has found NR could actually increase the risk of serious disease, including developing cancer. The international team of researchers led by Elena […]
To address these important research gaps, we conducted a small ... The remaining sample was slowly added to a new 50 ml conical tube containing 10 ml of ... Nicotinamide riboside and doubly labeled nicotinamide riboside (13C1, H 2-1) were obtained from ChromaDex Inc. (Irvine, CA). All HPLC solvents and extraction solvents were HPLC grade or ...
ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD +) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted ...
The data suggest that NR's acute cardioprotective effects are mediated through glycolysis activation and are lost in the presence of insulin because of already elevated glycolysis. Decreased nicotinamide adenine dinucleotide (NAD+) levels contribute to various pathologies such as ageing, diabetes, heart failure and ischemia-reperfusion injury (IRI). Nicotinamide riboside (NR) has emerged ...
Setting the benchmark as the gold standard in scientific rigor, safety, quality, and transparency, ChromaDex is the innovator behind its clinically proven flagship ingredient, Niagen ® (patented nicotinamide riboside, or NR), the most efficient and superior-quality NAD + booster available.
CDXC ChromaDex Corporation ChromaDex Receives Exclusive U.S. FDA Orphan Drug Designation (ODD) and Rare Pediatric (RPD) Disease Designation for Nicotinamide Riboside Chloride (NRC) for the Treatment
NAD + is synthesised from sources that include NMN, tryptophan, nicotinic acid, nicotinamide riboside and nicotinamide. NAD + precursors are found in small amounts in natural foods, such as cow ...
A six-month-old infant presented with riboflavin unresponsive lactic acidosis and life-threatening cardiomyopathy. Treatment with high dose bezafibrate and nicotinamide riboside resulted in marked clinical improvement including reduced lactate and NT-pro-brain type natriuretic peptide levels, with stabilized echocardiographic measures.
ChromaDex plans to conduct additional studies on NRC in anticipation of filing for an Investigational New Drug Application (IND) for the treatment of AT ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD + ) and healthy aging research, today announced that the U.S. Food Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric ...
Nicotinamide riboside (NR) is in wide use as an NAD+ precursor vitamin. Here we determine the time and dose-dependent effects of NR on blood NAD+ metabolism in humans. We report that human blood ...
LOS ANGELES, June 07, 2024--ChromaDex Corp. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD+) and healthy aging research, today announced that the U.S. Food & Drug ...
Nicotinamide riboside (NR) is a newly discovered nicotinamide adenine dinucleotide (NAD+) precursor vitamin. A crystal form of NR chloride termed NIAGEN is generally recognized as safe (GRAS) for ...
LOS ANGELES--(BUSINESS WIRE)-- ChromaDex, Inc. (NASDAQ:CDXC), the global authority on nicotinamide adenine dinucleotide (NAD +) and healthy aging research, today announced that the U.S. Food & Drug Administration (FDA) granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) Designation for NRC, the company's product candidate for the treatment of Ataxia Telangiectasia (AT).