Systematic Review: Autism Spectrum Disorder and the Gut Microbiota
Information & authors, metrics & citations, view options, conclusion:, introduction, search strategy, selection citeria, box 1. summations, limitations, study selection.
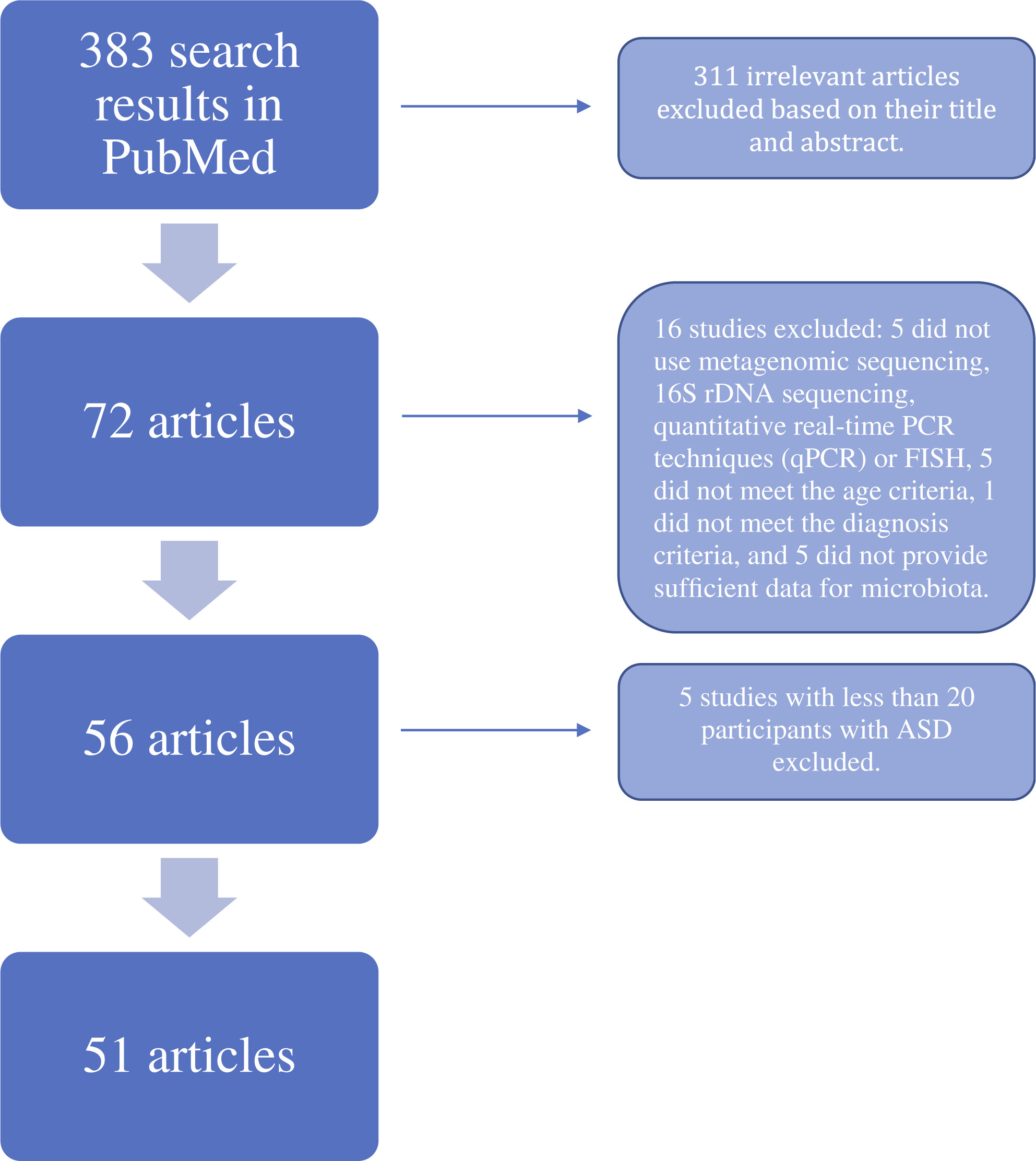

Study Characteristics
Alpha and beta diversity.
| | Result | Number of studies | References |
|---|---|---|---|
| α-diversity | ASD > control group | 8 | – , , , , , |
| | ASD < control group | 7 | , , , , , , |
| | No significant group difference | 19 | – , , , , , , , , , , , , – , |
| β-diversity | Gut microbiota composition significantly different between ASD and control groups | 26 | – , – , – , – , – , , , – , |
| | No significant group difference | 2 | , |
Specific Phyla and Genus Level Differences
| Taxonomic rank | Taxa | Abundance in ASD participants compared to controls | Number of studies | References |
|---|---|---|---|---|
| Phylym | Firmicutes/Bacteroidetes Ratio | ↑ | 5 | , , , , |
| | | ↓ | 3 | , , |
| Phylym | Firmicutes | ↑ | 2 | , |
| | | ↓ | 2 | , , |
| Class | Bacilli | ↑ | 2 | , |
| Genus | Lactobacillus | ↑ | 4 | , , , |
| | | ↑ after intervention | 1 | |
| Family | Lachnospiraceae | ↑ | 1 | |
| | | ↓ | 3 | , , |
| | | ↑ after intervention | 1 | |
| Genus | Coprococcus | ↓ | 2 | , |
| Genus | Roseburia | ↓ | 2 | , |
| Genus | Clostridium | ↑ | 2 | , |
| | | ↓ after intervention | 1 | |
| Species | Clostridium perfringens | ↑ | 3 | , , |
| Genus | Faecalibacterium | ↑ | 2 | , |
| | | ↓ | 3 | , , |
| Genus | Oscillospira | ↑ | 2 | , |
| Genus | Ruminococcus | ↑ | 3 | , , |
| | | ↓ | 3 | , , |
| | | ↑ after intervention | 1 | |
| Genus | Dialister | ↑ | 2 | , |
| | | ↓ | 2 | , |
| Genus | Veillonella | ↑ | 1 | |
| | | ↓ | 2 | , |
| Phylym | Bacteroidetes | ↑ | 2 | , |
| | | ↓ | 5 | , , , , |
| Genus | Bacteroides | ↑ | 4 | , , , |
| | | ↓ | 4 | , , , |
| Genus | Prevotella | ↑ | 3 | , , |
| | | ↓ | 3 | , , |
| Genus | Prevotella | ↑ | 3 | , , |
| | | ↓ | 3 | , , |
| Phylym | Proteobacteria | ↑ | 5 | , , , , |
| | | ↑ | 1 | |
| Order | Enterobacteriales | ↑ | 2 | , |
| Family | Enterobacteriaceae | ↑ | 4 | , , , |
| | | ↓ | 1 | |
| Genus | Escherichia/Shigella | ↑ | 4 | , , , |
| | | ↓ | 1 | |
| Species | ↓ | 2 | , | |
| Genus | Sutterella | ↑ | 4 | , , , |
| Genus | Phyllobacterium | ↑ | 2 | , |
| Genus | Flavonifractor | ↓ | 2 | , |
| Phylym | Verrucomicrobia | ↓ | 2 | , |
| Phylym | Actinobacteria | ↑ | 4 | , , , |
| | | ↓ | 1 | |
| Genus | Bifidobacteria | ↑ | 4 | , , , |
| | | ↓ | 4 | , , , |
| | | ↑ after intervention | 2 | , |
| Genus | Nitriliruptor | ↑ | 2 | , |
| Genus | Collinsella | ↑ | 3 | , , |
| | | ↓ | 1 |
Intervention Outcomes
Functional characteristics, conclusions, information, published in.

- autism spectrum disorders (ASD)
- gut microbiota
- gut-brain-axis
- systematic review
Affiliations
Competing interests, export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
PREVIOUS ARTICLE
Next article, request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Autism spectrum disorders
A review of the literature from arab countries.
Hussein, Hanan; Taha, Ghada R.A.
Department of Child Psychiatry, WHO Collaborative Center for Training and Research, Institute of Psychiatry, Ain Shams University Hospitals, Cairo, Egypt
Correspondence to Hanan Hussein, MS, MD, Department of Child Psychiatry, WHO Collaborative Center for Training and Research, Institute of Psychiatry, Ain Shams University Hospitals, Abbasseya, PO Box 11657, Cairo, Egypt E-mail: [email protected]
Received July 28, 2012
Accepted February 19, 2013
Background
In the developing countries and specifically in the Arab world, the field of child psychiatry is relatively new. Autism became a subject of interest in the region only during the late 1990s. Tremendous effort is needed to raise the awareness of policy makers toward the need for implementation of services and research plans aiming to bridge the gap between the needs of and services provided for autism.
The aims of the study were to: (a) focus on the magnitude of the problem of autism in the Arab world; (b) highlight research conducted in this field; and (c) shed light on services provided for autistic patients and their families in the Arab world with special focus on cultural and economic characteristics of the region and its impact on autism.
Methods
All internationally published English language articles and their reference lists were reviewed using MEDLINE (1992–2012) and studies on autism spectrum disorder conducted in all Arab countries were selected.
Results
Data from studies conducted by different Arab countries on various fields of autism research – genetic, autoimmune, oxidation stress, nutritional deficiencies, environmental toxins, errors of metabolism, mitochondrial dysfunction, clinical studies, imaging, treatment outcome, and available services for autistic patients – were collected and reviewed. Between 1992 and 2012 a total of 75 articles were published from Arab countries, most of which ( n =55, 73.3%) were published in the last 4 years. Most of the articles discussed possible etiologies of the disorder ( n =42, 56.6%). Autoimmune theory was the most commonly investigated in the Arab region ( n =12, 16%). Review articles constituted 16% ( n =12) of the published articles. The least addressed topic was treatment outcome ( n =4, 5.3%). Most of the research came from Saudi Arabia ( n =23, 30.6%) and Egypt ( n =16, 21.3%). Lebanon came third ( n =11, 14.7%) and had mostly review articles ( n =6). Internationally published studies on the prevalence of autism in Arab countries showed inconsistent frequency.
Conclusion
There is growing interest in autistic disorders in the Arab world, particularly in Egypt and Saudi Arabia, as evidenced by the increase in research in this field in the last few years. Research on autism in Arab countries is still in its infancy and needs organizational efforts to be fruitful. Further research is needed within Arab countries to determine the most effective and efficient means of improving diagnosis and service delivery in the context of the particular country, culture, and governmental structure.
Introduction
Research is an important means of finding effective ways to lessen the burden of disease. Most research into autism spectrum disorder (ASD) has been conducted in affluent English-speaking countries, which have extensive professional support services 1 . Initial research on autism diagnosis and service delivery in developing countries has suggested that diagnosis can be a cumbersome and frustrating process. Access to effective therapeutic services is often limited or nonexistent, and the greater part of the burden of care rests on family members 2–4 . Publications on child psychiatry in the Arab region, particularly in the field of autism, were found to be under-represented 5 . This shortage of information has raised the unwarranted assumption that autism is rare in non-western cultures 6 .
The authors have previously published an overview of the condition in the Arab world in which a holistic approach to autism was observed. Nevertheless, the authors by the end of their work found out that, in addition to being scanty and unorganized, there were no data available for the state of autism in the Arab region collectively. In most cases data are presented for each country separately, which poses a major obstacle to proper administration of service to this group of patients in the region 7 . We believe that research-policy makers should consider autism research as a priority in the Arab region if they intend to improve the recognition of and services offered to autistic individuals in their countries. Accordingly, we decided to bring forth a detailed article on the research condition in the field of autism in the Arab world with respect to its prevalence, clinical profile, etiology, services provided, and outcome.
The authors thus aimed to gather all available articles published on autism in the Arab countries and analyze them in order to help highlight which areas of research need to be adopted in the near future.
The aims of this work were to: (a) focus on the magnitude of the problem; (b) highlight research conducted in this field; and (c) shed light on the services provided for autistic patients and their families in the Arab world with special focus on cultural and economic characteristics of the region and its impact on autism.
A review of the published work according to the PRISMA statement 8 was conducted. Relevant studies were identified by searching Medline via Pubmed (from 1992 to May 2012). The following keywords were used in the search: ‘autism’, ‘autistic disorder’, ‘asperger’, ‘Egypt’, ‘Libya’, ‘Tunisia’, ‘Algeria’, ‘Morocco’, ‘Mauritania’, ‘Sudan’, ‘Somalia’, ‘Djibouti’, ‘Lebanon’, ‘Jordan’, ‘Gaza’, ‘Syria’, ‘Iraq’, ‘Saudi Arabia’, ‘Kuwait’, ‘Qatar’, ‘Bahrain’, ‘United Arab Emirates’, ‘Oman’, ‘Yemen’. The reference lists of these papers were searched for additional articles. All identified articles were reviewed for eligibility by both authors. Publications with a comprehensive abstract or full text were included. Publications in local journals and periodicals were excluded because of difficulty in accessing all national periodicals and the full-text articles published in them.
Both authors independently extracted data from selected articles using a specially designed data extraction form that included the name of the study, the date of publication, country of research, scope of research, number of patients included, and main results. Data were then entered in SPSS, version 17 (IBM Corporation, New York, USA) for further analysis.
A total of 79 articles were published from all Arab countries from 1992 to 2012, most of which ( n =58, 73.4%) were published in the last 4 years. Most of the research came from Saudi Arabia ( n =25, 31.6%) and Egypt ( n =16, 20.25%). Lebanon came third ( n =11, 13.9%) ( Table 1 ). Most of the articles discussed possible etiologies of the disorder ( n =42, 56.6%). Autoimmune theory was the most commonly investigated in the Arab region ( n =12, 15.1%). Review articles constituted 17.7% ( n =14), many of which were from Lebanon ( n =6, 7.5%). The least addressed topic was treatment outcome ( n =4, 5%) ( Tables 2 and 3 ).

Research on prevalence
The contribution of different Arab countries to international prevalence studies of ASD is scarce. One report from Saudi Arabia estimated that there were 42 500 confirmed cases of autism in 2002 and that many more remained undiagnosed 9 . This translates to about 18/10 000 cases of autism 5 . Another field study conducted in Saudi Arabia for 3 years, using the Arabic version of CARS, reported that there were 57 110 children younger than 16 years of age with ASD. Most cases were found outside Riyadh, giving a prevalence of 0.6% with a ratio of ∼1 : 2 for girls to boys 10 . In the Sultanate of Oman, prevalence was 1.4 cases per 10 000 children 11 , whereas in the United Arab Emirates (UAE), from a representative random sample of 3-year-old UAE national children, 29 per 10 000 children had autism 12 .
Other studies tried to determine the frequency of autism in clinical samples. In Jordan, 5.2% ( n =12) of a clinical sample of children with Global Developmental Delay ( N =229) were found to be autistic 13 . In Libya, a total of 38 508 children were seen in the pediatric clinic of KH, Tripoli, of which 128 children were autistic. This gives a frequency rate of one in 300 14 . The prevalence of ASD among children with developmental disorders in Egypt and Tunisia was documented as 33.6 and 11.5%, respectively 15 . According to the CAPMS-ARE, roughly two million Egyptians are disabled or have special needs, of which 1 515 100 are mentally handicapped 16 . A simple calculation, as used by Seif Eldin et al . 15 , reveals that in Egypt as many as 509 073 children might be affected by autism.
Research on etiology
As mentioned before, most of the research coming from the Arab world was concerned with the possible etiologies of autism.
Genetics etiology research
Ten articles were published on genetics in the region. However, the sample size was generally too small, and in many cases published articles were case reports ( Table 4 ).

Autoimmune etiology
Twelve articles were published on this topic in the Arab world, with Egypt and Saudi Arabia publishing an equal number of articles, mostly within the past 3 years ( Table 5 ).

Hormonal disturbances research
Only one study was reported internationally from Egypt. It was carried out on 50 patients with autism. It revealed that 16% of autistic patients had high adrenocorticotrophic hormone (ACTH) levels, 10% had low basal cortisol levels, and 10% did not show adequate cortisol response to ACTH stimulation. Autistic patients had lower basal ( P =0.032) and stimulated cortisol ( P =0.04) levels and higher ACTH ( P =0.01) levels compared with controls. CARS scores correlated positively with ACTH level and negatively with basal and stimulated cortisol levels. The hormonal profile did not differ in relation to electroencephalogram abnormalities, IQ, and self-aggressive symptoms 37 .
Nutritional deficiencies and errors of metabolism
The majority of the internationally published research was from Egypt and Saudi Arabia. Egyptian children with autism showed significantly lower 25(OH)D and 1,25(OH)(2)D levels as well as lower calcium serum values compared with controls. A significant positive correlation was obtained between 25(OH)D and calcium levels in children with autism. The researchers concluded that serum values of 25(OH)D in children with autism in this study could classify them as being ‘vitamin D inadequate,’ which lends support to the hypothesis that autism is a vitamin D deficiency disorder 38 . Another study from Saudi Arabia showed remarkable alteration in polyunsaturated fatty acids (PUFA) and omega-3/omega-6 and significantly lower levels of phospholipids among autistic children 39 . Another Omani study found that mean serum Hcy levels were significantly ( P <0.05) higher in autistic children (20.1±3.3 µmol/l) as compared with controls (9.64±2.1 µmol/l). Significantly ( P <0.05) lower serum folate (1.8±0.4 µg/l) and vitamin B 12 (191.1±0.9 pg/ml) levels were observed in autistic children as compared with controls (6.1±0.6 µg/l and 288.9±1.3 pg/ml, respectively). A high serum homocysteine (Hcy) level is regarded as an indicator of impairment of the folate-dependent methionine cycle and is associated with oxidative stress, as reported in an Omani study. The levels of homocysteine in autistic children were also much higher as compared with normal reference values (5–15 µmol/l). The results suggest that high fasting serum homocysteine and low folate and vitamin B 12 levels could be used as clinical biomarkers for early diagnosis and management of ASD 40 .
Environmental toxins research
Three studies from Saudi Arabia and one from Kuwait investigating the effect of environmental toxins on autistic patients were published internationally. Studies from other Arab countries in this issue are lacking ( Table 6 ).

Mitochondrial dysfunction and oxidative stress
Seven studies from Egypt, Saudi Arabia, and the Sultanate of Oman were internationally published in this issue (Table 6).
Research on clinical status
There were only a few studies published internationally in this field, and they were mainly from Saudi Arabia, Egypt, UAE, and Jordon. In a clinical study from Saudi Arabia investigating 49 patients with autism (37 male and 12 female patients), female patients were older than male patients at the time of referral; 11 patients had a history of seizure disorder, and one patient had a chromosome abnormality. Twenty-five patients were taking psychotropic medications and 14 patients were the product of consanguineous marriages. Communication deficits were the most common cause for referral of Saudi autistic children. Hyperactivity and aggression were reported in 44.8% of Saudi patients; epilepsy was found in 22.4% and almost half of them were referred for co-occurring behavioral problems, particularly hyperactivity and aggression. The researchers argued that the higher incidence of epilepsy in the Saudi group may be attributed to higher genetic loading and more developmental and perinatal problems 2 , whereas in the UAE the presence of autistic features was associated with male gender, presence of behavioral problems, and a family history of developmental delay. Also, the rate of pervasive developmental disorders observed in the UAE is comparable to that reported from western countries 12 . From Jordan, a study reported that motor and tactile-perceptual skills (grip strength, motor speed, and coordination) were impaired in individuals with high-functioning autism when compared with matched normally developing individuals 51 . In Egypt, a study was conducted to determine the possible risk factors of autism in 100 Egyptian autistic patients recruited from a pediatric hospital at Ain Shams University; 46% of patients presented at the age of 1.5 years and 32% at the age of 2 years. Moreover, 55% of patients had mild to severe retardation. High maternal age at birth was found in 23% of autistic children. Also, advanced paternal age at birth was found in 91% of cases. Positive family history was found to be significantly associated with the risk of autism (16% of cases vs. 1% of controls). Postnatal factors such as history of hypoxia, resuscitation, and history of jaundice were considered significant risk factors for autism 52 .
In contrast, there were only five studies comparing clinical status across two or more Arab countries simultaneously. With the apparent increase in the prevalence of ASD in Arab countries, the development of an Arabic tool for early diagnosis and intervention became essential to better understand the prevalence of this disorder. Accordingly, a study was conducted in nine Arabic speaking countries aiming to validate M-CHAT as an Arabic tool for the screening of autism. The final analysis included 228 children (122 of whom screened positive for ASD). The new translated tool showed acceptable reliability and validity. Maternal health problems during pregnancy and labor were more significant for ASD mothers than for controls. Pediatric health problems were significantly more evident among ASD patients than among controls 15 . Another recent study investigated 37 boys and 23 girls from three Arab countries (Egypt, Saudi Arabia, and Jordan). The results showed that boys had poor emotional responsiveness and girls had more cognitive problems. Boys exhibited significantly more delinquent behavior 53 . The third study investigated and compared patients with autism in Egypt ( n =20) and Saudi Arabia ( n =28) with respect to both demographic and clinical characteristics. Patients in both groups had typical autism rather than atypical autism, with regression reported in 30% of Egyptian patients and 21.4% of Saudi patients. Fifty percent of Egyptian patients showed hyperactivity as compared with 60.7% of Saudi patients. Five percent of Egyptian patients suffered from epilepsy in comparison with 25% of Saudi patients. Psychiatric comorbidities were reported in 71.4% of Egyptians and 67.5% of Saudi patients. Egyptian autistic patients were characterized by delayed language development, earlier age at start of treatment intervention, high preference for behavioral and phonetic therapies, higher paternal and maternal education, higher employment among parents, and higher family concern. In contrast, Saudi patients were characterized by delay in all developmental milestones, severe and profound communication defects, more stereotypes and developmental deficits, younger age at detection of abnormality and older age at start of treatment intervention, with marked difference between the two (being around 2 years), higher percentage of missing examinations, older birth order, and significantly higher preference for drug treatment. The study attributed the reported differences between Egyptian and Saudi autistic patients to cultural factors and differences in awareness of autism 54 .
Only one preliminary study tried to determine the general outcome of Egyptian and Saudi autistic children after 2 years of follow-up. It revealed a nonstatistically significant tendency toward better outcome in Egyptian autistic patients compared with Saudi patients. This tendency is revealed in the form of improvement in the Gilliam severity of autism in Egyptian children and worsening of Vineland scores in Saudi children. Good outcome was generally associated with higher age at detection of abnormality, high IQ, mild severity of autism, high Vineland scores, low stereotype scores, atypical autism, absence of seizures and regression, and high parental concern 54,55 . An interesting study that was published recently investigated a possible association between psychiatric comorbidities and autism in Egypt, Saudi Arabia, and Jordan. It was found that 63% of children were diagnosed with at least one comorbid disorder. The most commonly reported comorbid disorders were anxiety disorders (58.3%), ADHD (31.6%), conduct disorders (23.3%), and major depressive disorder (13.3%). Out of the total sample, obsessive compulsive disorder was the most prevalent anxiety disorder (55%). Elimination disorders were also diagnosed in 40% of patients of patients 56 .
Research on imaging
Imaging studies are scarce in the Arab region. Thus far, only one Egyptian study has been published internationally. It shows significant decrease in the medial prefrontal cortex bilaterally and in the left anterior cingulate cortex in autistic children. Regression analysis revealed a positive correlation between the medial prefrontal cortical thickness and social IQ 57 .
Research on treatment outcome
Three studies on this issue were published internationally from Egypt, Kuwait, and Lebanon. The Egyptian study was conducted on 30 autistic children (18 boys and 12 girls) aged 3–11 years; 30 healthy children comprised the control group. This study reported that, before taking Efalex, autistic patients showed a significant reduction in linolenic acid levels (71%), followed by docosahexaenoic acid (65%) and arachidonic acid (45%) levels, whereas linoleic acid was the least affected PUFA (32%). After taking Efalex, 66% of autistic children showed clinical and biochemical improvement. Linolenic acid and docosahexaenoic acid showed the highest levels after Efalex supplementation. The results imply that PUFA supplementation may play an important role in ameliorating autistic behavior 58 . The Kuwaiti study was carried on 40 autistic patients over a period of 13 weeks. It showed that olanzapine treatment can be beneficial in alleviating some behavioral symptoms (irritability, hyperactivity/noncompliance, and lethargy/withdrawal) associated with autism. The short period of this trial limits inferences about adverse effects such as body weight increase and tardive dyskinesia 59 . The Lebanese study was carried out on Lebanese autistic children in the USA. It showed statistically significant improvements in correct responses following exercise in open air. No significant differences were found for on-task behavior or stereotypic behaviors 60 .
Only one study was carried out on caregivers in the Arab world: this study was conducted in Qatar. It compared the caregivers of autistic children between 3 and 17 years of age with caregivers of normally developing children. There was no significant difference in the quality-of-life domains between the two groups of caregivers, but caregivers of autistic children rated their health as poor and likely to get worse. This study provided some evidence for the impact of caring for a child with autism on the life of the caregiver. The findings should help health-policy makers in Qatar to provide better and more focused support to children with autism and to their caregivers 61 .
Research on services available and support for autism spectrum disorder families
Research in this field is available only from Saudi Arabia and Egypt. In Saudi Arabia, access to early intervention services is limited to the major cities of Riyadh, Jeddah, and Al-Dammam. Almassoud 62 in his study stated that services are provided mainly by the private sector, which is unable to meet the increased demand on services. Local and public services are not available to support the parents of autistic children and provide the required knowledge and help in Saudi Arabia. Al-Othman 63 reported the recent establishment of an autism research and treatment institute related to King Fahd University, and across the Kingdom of Saudi Arabia there are three centers specializing in autism – the Academy of Special Education, Jeddah Centre for Autism, and Prince Faisal bin Fahd mother’s Centre. All of these centers adopt Treatment and Education of Autistic and Related Communication Handicapped Children (TEACCH) as a comprehensive educational program for all children of school age. One of the problems of using TEACCH in all of these centers is that it is not suitable for all autistic children, especially for children with high-functioning autism 62,63 .
In Egypt, services are usually clustered in large cities such as Cairo and Alexandria. They provide speech therapy, cognitive training, and skill-oriented behavioral modification. A few might provide psychomotor training. It is worth mentioning that those services are available at a governmental or nongovernmental level. The Egyptian state plays a restricted role in healthcare provision, particularly with respect to developmental/learning disabilities. According to Mendoza 4 , few families used ASD interventions or institutionalized autism-related services, citing lack of knowledge about ASD and effective interventions, limited state or community resources, limited financial resources, and lack of faith in the services. He reported that the more urbanized communities tend to have greater access to, and hence utilize, health and welfare resources. In contrast, in rural areas, virtually every individual with ASD stays home with his/her family or extended family network. Thus, parents in Egypt and in many developing countries are reliant on informal sources of support, as professional services are often poorly represented with lack of training and expertise in ASD among the available staff, doctors, teachers, or therapists 64 .
Special education schools for children with learning disabilities and mental retardation have been established in Egypt, Jordan, Lebanon, Saudi Arabia, Tunisia, and the UAE 65 . In terms of vocational rehabilitation and employment support for adults with autism, there are no current training colleges or organizations that support autistic adults across the Arab world. However, in some countries like Saudi Arabia, the Saudi Autistic Society has established a new summer program for adults with autism. This program provides training in computer skills and other technical skills 62 . Unfortunately, it is limited to the period of summer vacation and does not offer a wide variety of training modules. In countries like Egypt, the extended family network offers autistic Egyptians some advantages in terms of old-age income and support, which may not be easily available to their counterparts in industrialized countries 4 .
Arab organizations for autism
National organizations for children and families with autism now exist in different Arab countries. These organizations provide workshops, courses, and lectures to elucidate vague aspects of autism aiming to increase public awareness 62 . In Saudi Arabia, although considerable voluntary effort is provided by different charity sectors, there is no clear volunteering system, nor is there information available in the Saudi Autistic Society website 66 . In Egypt, few organizations exist that meet the special needs of people with ASD and other developmental/learning disabilities (e.g. The Egyptian Autistic Society, Learning Resource Center, and others). Most of them are private and nonprofit organizations that possess the relevant expertise and experience. However, the institutional capacities of these few organizations are extremely limited because of underfunding 4 .
Research on economic costs of autism
Research on the economic costs of autism in developing or semi-industrialized countries including Arab countries is virtually nonexistent. Only one study tried to address this issue in Egypt 4 using survey data gathered from a probability sample of 165 households in the Greater Cairo Region. Mendoza found that 91.2% of individuals with ASD relied on immediate family for care. Direct financial cost estimates of autism care range from LE 171 368 to 251 303 (US$31 300–45 900). Most of the expenditure is for nonmedical items such as special education, skills training, or camps rather than for medical services, prescription medication, or behavioral therapy. As for indirect costs of caring for an ASD member, Mendoza reported that the median extra time spent by surveyed family caregivers on autistic individuals was ∼1211–1239 h a year. Also, lost income, productivity, and leisure time constitute the indirect cost consequences of ASD for individual caregivers in Egypt. However, the economic impact of these losses could vary significantly depending on the particular economic and social circumstances of the caregiver.
Our results showed that autism in most Arab countries except for Egypt and Saudi Arabia is not yet a priority, neither with respect to research nor with respect to services. This might be because the field of child psychiatry is relatively new in these countries 6 . According to our results, prevalence studies are lacking in this region. This might be because of the lack of funding in economically burdened countries like Egypt 4 or because of lack of concern for research policy in other countries 67 .
The difference in the clinical profile of patients between countries might be attributed to cultural factors, which play an important role in shaping some dimensions of the illness behavior with respect to symptom recognition and response to illness. Arab cultures are generally characterized by the high rate of consanguineous marriages, high support for nuclear families by their extended families, and consequently high tolerance rate for taking care of mentally ill individuals, especially children. Arabic cultures and families are more tolerant to behaviors in children that would be seen by western societies as ‘abnormal’. However, any claims remain speculative as little or no research has been carried out to investigate the effects of culture on autism 64 .
A lot remains to be learned about autism-related costs in developing countries. Worldwide, families bear a significant portion of the financial burden of ASD, with virtually all of it being borne by families in developing countries. The portions of the caregiving burden that the family cannot sustain will inevitably spill over to society in one form or another 1 . The direct estimates reported by Mendoza were considerably lower than similar estimates obtained in developed countries. The direct nonmedical costs, along with some physician and outpatient services, accounted for the higher proportion of cost in Egyptian families, which would bear about 79.0–88.3% of these costs in the absence of private health insurance coverage or aid for developmental/learning disabilities. In contrast, indirect costs of autism in Egypt were significantly higher compared with countries like Sweden, where the estimated annual family expenditure of extra time amounts to about 1000 h 68 .
Most of the research in Arab countries was concerned with etiology of autism rather than with services and treatment outcome. This might indicate that medical fields are far more developed than educational and rehabilitation fields with respect to this type of disorder. It raises the awareness of policy makers about the need to implement measurable evidence-based services in their countries, which also abide by regulations for documentation and supervision to prevent malpractice. Further, there are more studies on autoimmune and chemical biomarkers of the disorders than there are on imaging and genetics. This might reflect the difficulty of this type of research, which needs extensive funding beyond the capabilities of individual researchers. In addition, we found that results of etiology studies did not seem different from those of western countries, which indicate that no specific environmental or ethnic factors have been proved to cause autism yet. This is in accordance with previous research by Bristol et al. 69 . Genetic studies are probably the most desperately needed. It is evident that most genetic studies in the region are just case reports or with very limited sample size. Large sample studies are, however, essential to prove or disprove these reported findings. Recently, there has been growing interest in the region in genetic research because of the specific cultural characteristics of the Arab community, especially in Saudi Arabia. Al-Salehi et al . 2 found that almost one-third of a cohort of Saudi children with autism had a history of consanguinity. Most consanguineous marriages in Arab countries are between first cousins. They can be quite prevalent, ranging from 34 to 80% of all marriages in Saudi Arabia, depending on location – higher in rural communities than in urban and suburban settings. Although the findings from Saudi Arabia do not directly link consanguinity with autism in Arab countries, they suggest that there is a higher incidence of autism among families in Saudi Arabia, making them ideal candidates for screening studies for any genetic variations with the aim of implicating biological processes. Accordingly, Walsh’s team turned to the Middle East due to increase in odds of finding rare genes. They recruited 88 families with consanguineous marriages and a high incidence of autism from Jordan, Saudi Arabia, Kuwait, Oman, Pakistan, Qatar, Turkey, and the UAE. They compared the DNA of family members to search for recessive mutations. In some of the families, they found large chunks of missing DNA regions that followed the recessive rule. The missing regions varied among families, but they affected at least six genes that play a role in autism 70 .
Imaging studies are very scarce in Arab countries. A possible explanation might be the procedural difficulty in carrying out this type of research, as patients in most cases need to be anesthetized. This does not mean that patients do not undergo imaging studies. They often come to the center carrying a report from an MRI or computed tomography scan that they had undergone some time back, but lack of well-planned pathways to services and lack of documentation lead to loss of data in most cases.
Currently, there is growing interest in autistic disorders in the Arab world, particularly in Egypt and Saudi Arabia, as evidenced by the increasing research in this field in the last few years. Scientists should highlight the magnitude of the problem and raise public awareness as well as direct the attention of policy makers to the need to implement evidence-based services for this group of patients, which is drastically needed. Research on autism in Arab countries is still in its infancy and needs organization efforts to be fruitful. Further research is needed to determine the most effective and efficient means of improving diagnosis and service delivery in the context of a given country, culture, and governmental structure.
Strengths and limitations
To the best of our knowledge, this is one of the first studies to comprehensively investigate the problem of ASD in the Arab world. This article reviewed all internationally published studies from different Arab countries in different aspects of ASD. However, the main limitation that faced authors was the difficulty in accessing studies published in different national periodicals in different Arab countries. This limited our review to only internationally published articles.
Recommendations
At the governmental level, the relevant governmental agencies should provide and ensure affordability of and accessibility to autism resources, including educational services for autistic children and their caregivers. The various ministries of higher education should initiate training programs for teachers to educate them on the appropriate skills needed to give every child with autism the best care. More importantly, there is a desperate need for national policy formulation to care for autistic children in different Arab societies.
At a professional level, there is a marked need to train pediatricians in the core features of autism and relevant differential diagnosis and management options. Initiatives for early detection and intervention are considered medical priority. It is necessary to include diagnosis and management of autism in the curriculum of medical schools. At present, there is lack of focus on psychiatric education across the Arab world, specifically pediatric psychiatric disorders.
At the caregiver level, caregivers should be involved in planning and evaluating autism policies and programs, such as school integration. Family training and education is important in passing knowledge and experiences to others through informal contacts. The formation of local associations is very important for promoting parental empowerment and for providing formal support services to assist the children and their families.
At the research level, a research foundation is needed to integrate research efforts from different Arab countries for standardization of screening and diagnostic tools across countries and cultures. Extensive research is needed to determine management recommendations and treatment modalities that best suit various Arab cultures.
At the level of organizations, more centers for treating autism are needed. The current autism centers have to increase their capacity to accept more children in their programs and adopt various educational programs. Special schools for severe conditions are also needed. Vocational rehabilitation for adults with autism is also needed to minimize the costs on families and caregivers. This can be achieved by establishing training colleges and organizations specialized in vocational rehabilitation for raising employers’ awareness about the working ability of autistic people.
Finally, the public and private sectors have to cooperate to ensure that more organized and effective efforts are provided.

Acknowledgements
Conflicts of interest.
There are no conflicts of interest.
- Cited Here |
- PubMed | CrossRef
- View Full Text | PubMed | CrossRef
- View Full Text | PubMed
Arab countries; autism; autistic disorder; research; services
- + Favorites
- View in Gallery
- Introduction
- Conclusions
- Article Information
FR indicates face recognition.
Meta-analysis forest plot of all unique studies (N = 38) depicting the mean difference in the FIE between autistic and neurotypical participants (Hedges g ) for each study as represented by a posterior distribution. Individual points represent individual effect sizes nested within study. The overall summary effect shows the mean effect size of the FIE difference between autistic and neurotypical participants across all studies. Colors differentiate studies from each other. Values on the right side of the plot reflect the mean (Hedges g ) and 95% credible intervals (CrIs) for the study-level posterior probability distributions. Two effect size values of −19.33 and −8.10 are not represented in this figure to avoid obscuring the figure.
Differences in the magnitude of the summary effect size as a function of methodological factors, including psychological construct, measurement modality, and recognition demand, are shown on the left. Negative values suggest reduced face inversion effect (FIE) in autism and positive values suggest an increased FIE in autism. Point intervals reflect 95% quantile intervals. How the summary effect size is associated with sample-related factors, including mean sample age, sample sex distribution (% male), and average sample IQ score, are shown on the right. Dashed horizontal line represents the overall summary effect size (Hedges g = −0.41), whereas the solid line (with 95% CI) represents a fitted regression line. Point color differentiates individual studies. Two effect size values of −19.33 and −8.10 are not represented in this figure to avoid obscuring the figure. ASD indicates autism spectrum disorder; EEG, electroencephalogram.
eTable. PRISMA Checklist
eTable 2. PRISMA Abstract Checklist
Data sharing statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Griffin JW , Azu MA , Cramer-Benjamin S, et al. Investigating the Face Inversion Effect in Autism Across Behavioral and Neural Measures of Face Processing : A Systematic Review and Bayesian Meta-Analysis . JAMA Psychiatry. 2023;80(10):1026–1036. doi:10.1001/jamapsychiatry.2023.2105
Manage citations:
© 2024
- Permissions
Investigating the Face Inversion Effect in Autism Across Behavioral and Neural Measures of Face Processing : A Systematic Review and Bayesian Meta-Analysis
- 1 Yale Child Study Center, Yale University School of Medicine, New Haven, Connecticut
Question Do autistic individuals show a face inversion effect?
Findings This systematic review and bayesian meta-analysis of 38 studies comparing face processing of upright and inverted faces in autistic and neurotypical individuals found reduced face inversion effect in autistic individuals.
Meaning This reduced face inversion effect in autism suggests less specialization or expertise of the face processing system, particularly in recognizing emotion from faces as measured in behavioral paradigms.
Importance Face processing is foundational to human social cognition, is central to the hallmark features of autism spectrum disorder (ASD), and shapes neural systems and social behavior. Highly efficient and specialized, the face processing system is sensitive to inversion, demonstrated by reduced accuracy in recognition and altered neural response to inverted faces. Understanding at which mechanistic level the autistic face processing system may be particularly different, as measured by the face inversion effect, will improve overall understanding of brain functioning in autism.
Objective To synthesize data from the extant literature to determine differences of the face processing system in ASD, as measured by the face inversion effect, across multiple mechanistic levels.
Data Sources Systematic searches were conducted in the MEDLINE, Embase, Web of Science, and PubMed databases from inception to August 11, 2022.
Study Selection Original research that reported performance-based measures of face recognition to upright and inverted faces in ASD and neurotypical samples were included for quantitative synthesis. All studies were screened by at least 2 reviewers.
Data Extraction and Synthesis This systematic review and meta-analysis was conducted according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. Multiple effect sizes were extracted from studies to maximize information gain and statistical precision and used a random-effects, multilevel modeling framework to account for statistical dependencies within study samples.
Main Outcomes and Measures Effect sizes were calculated as a standardized mean change score between ASD and neurotypical samples (ie, Hedges g ). The primary outcome measure was performance difference between upright and inverted faces during face recognition tasks. Measurement modality, psychological construct, recognition demand, sample age, sample sex distribution, and study quality assessment scores were assessed as moderators.
Results Of 1768 screened articles, 122 effect sizes from 38 empirical articles representing data from 1764 individual participants (899 ASD individuals and 865 neurotypical individuals) were included in the meta-analysis. Overall, face recognition performance differences between upright and inverted faces were reduced in autistic individuals compared with neurotypical individuals ( g = −0.41; SE = 0.11; 95% credible interval [CrI], −0.63 to −0.18). However, there was considerable heterogeneity among effect sizes, which were explored with moderator analysis. The attenuated face inversion effect in autistic individuals was more prominent in emotion compared with identity recognition ( b = 0.46; SE = 0.26; 95% CrI, −0.08 to 0.95) and in behavioral compared with electrophysiological measures ( b = 0.23; SE = 0.24; 95% CrI, −0.25 to 0.70).
Conclusions and Relevance This study found that on average, face recognition in autism is less impacted by inversion. These findings suggest less specialization or expertise of the face processing system in autism, particularly in recognizing emotion from faces as measured in behavioral paradigms.
Face perception, the ability to encode, discriminate, and recognize the faces of others, is a foundational social skill that develops within the first years of life 1 and relies on an extensive network of cortical and subcortical neural systems. 2 , 3 It is a necessary precursor for the development of social cognition 4 and is central to hallmark characteristics of autism. 5 Given the importance and centrality of faces to human development, humans develop highly specialized and efficient mechanisms for processing this class of social stimuli, but this specialization is specific to upright faces. The face inversion effect (FIE), the disproportionate performance cost associated with processing inverted compared with upright faces, is one of the most reliable markers of face expertise detectable across multiple measurement modalities (behavior, neural, and electrophysiological) and psychological constructs (identity and emotion recognition). For example, inverted compared with upright faces are more difficult to recognize, 6 - 8 produce reduced hemodynamic brain responses in the fusiform face area, 9 , 10 and generate significantly larger and slower electrophysiological brain responses. 11 - 13 This inversion cost is widely attributed to the disruption of the canonical configuration of facial features to which face processing mechanisms are attuned (eg, holistic processing). 6 , 14 , 15
Reduced face specialization in autism, including reduced use of configural or holistic processing, is cited as one mechanism by which autistic individuals struggle to recognize faces as efficiently and as accurately as their neurotypical peers. Reduced configural or holistic processing is generally supported by a reduced FIE in autism 16 - 18 ; however, these findings are not ubiquitous 19 and may systematically vary across measurement modalities, psychological constructs, recognition demands, and sample age groups. For example, when assessing behavioral task performance (accuracy), numerous studies do not find differential inversion effects between autistic and neurotypical individuals, 20 - 25 but these findings are not universal. 16 - 18 , 26 , 27 Similarly, differential neural response to upright and inverted faces as measured by the N170 event-related potential (eg, longer latency and larger amplitude to inverted compared with upright faces) or functional magnetic resonance imaging (reduced brain signal to inverted compared with upright faces) may be preserved in autism, 27 , 28 but numerous studies show otherwise. 29 - 31
This literature indicates that both behavioral and brain studies of the FIE in autism show heterogeneous results that have prevented conclusive determination of its presence in this population. The primary goal of this investigation was to systematically evaluate the factors that influence the magnitude of the FIE in autism. Given small sample sizes and methodological- and sample-related heterogeneity in published findings, we applied meta-analysis, moderation analysis, and meta-regression to synthesize and reconcile discrepant findings across the extant autism literature.
We used the following search parameters in the MEDLINE, Embase, Web of Science, and PubMed databases from inception to August 11, 2022: (face OR faces OR facial) AND (inversion OR inverted OR “upside down” OR rotation OR holistic OR configural) AND (autism OR autistic OR ASD OR Asperger). In adherence with transparent and rigorous psychological practices, 32 we identified, screened, and determined eligibility of empirical studies in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline, 33 including the content checklist (eTables 1 and 2 in Supplement 1 ) and study search flow diagram ( Figure 1 ). A backward citation search was conducted for all studies meeting inclusion criteria.
All articles must have (1) been written in English; (2) included an autism sample (eg, autism, Asperger syndrome, autism spectrum disorder [ASD], autistic disorder, pervasive developmental disorder–not otherwise specified); (3) included a neurotypical control sample free of any neurological or neuropsychiatric conditions (eg, typically developing, healthy control); (4) included a measure of face processing requiring participants to view, discriminate, perceive, or recognize face stimuli in upright (canonical orientation) and inverted (180° picture rotation of upright) conditions; and (5) reported statistical information necessary for calculating the effect size between upright and inverted conditions (eg, Cohen d , t , mean, SD, N, SE).
Articles were excluded if they (1) were not an empirical study (eg, book chapter, systematic review) or (2) did not provide necessary statistical or methodological information following email request.
A total of 7 reviewers (J.W.G., M.A.A., S.C.-B., C.J.F., N.H., R.I., and L.H.R.) double screened all entries throughout our multiphase (title/abstract, full text, and backward title, abstract, and full-text screening) screening approach using standardized abstract and full-text screening tools (eMethods in Supplement 1 ). Disagreements were resolved through an experienced reviewer (J.W.G.). Team feedback was provided throughout screening to prevent coder drift and improve reliability among all reviewers.
We extracted sample and methodological information used for moderator analysis (eMethods in Supplement 1 ) and group (ASD vs neurotypical) mean and SD values for upright and inverted conditions from the reported face recognition task paradigms. When not provided, they were calculated from other information (eg, SEs) or extracted from figures when appropriate. 34 Data were requested directly from corresponding authors via email when data extraction was not possible. We extracted multiple effect sizes from studies when available; for example, studies often reported multiple FIE measures from different types of stimuli (grayscale faces, color faces, Mooney faces), emotional expressions (happy, sad, fearful, angry), and measurement sites (left and right hemispheres). All extracted data were double screened for accuracy by all reviewers; disagreements were resolved through consensus.
We used a modified version of the Newcastle-Ottawa Scale 35 to assess the quality and risk of bias of included studies (eMethods in Supplement ). Each included study was coded by J.W.G. and C.J.K.; disagreements were resolved through consensus.
We calculated standardized mean change scores (upright – inverted) and the difference in standardized mean change scores (ASD – neurotypical) for each face recognition measure in each study 36 ; standardized mean change scores were converted to Hedges g to account for small-study bias (eMethods in Supplement 1 ). We analyzed all data using the brms version 2.17 and rstan version 2.21.5 packages for the statistical software program R version 4.2.1 (R CoreTeam). For all analyses (main effect and moderator models), we used a bayesian random-effects multilevel framework to account for nonindependence in effect sizes by using a 3-level model: sampling variance of observed effect sizes, within-study variability, and between-study variability. Given inconsistency in the direction and magnitude of the FIE, we specified a weakly informative prior distribution centered on zero [ N (0, 1)] to reflect our prior belief about the direction and magnitude of the FIE. For all variance parameters, we used a nonnegative half-Cauchy distribution [Cauchy (0, 0.3)] since variance estimates cannot be negative and the half-Cauchy distribution has desirable properties for psychological phenomena. 37 A bayesian modeling framework provides distinct advantages over frequentist methods, including (1) integration of prior information; (2) improved estimation of parameters and sources of variation; and (3) direct interpretation of parameter estimates via the posterior probability distribution (PPD). We quantified heterogeneity across all levels using proportional measures of variability ( I 2 ). 38 For all model coefficients, we reported 95% credible intervals (CrIs). We assessed small-study effects via funnel plot asymmetry by regressing the study-specific effect sizes onto their SEs (ie, modified Egger regression test) to account for the multilevel structure. Finally, we evaluated the presence of publication bias across study-averaged effect sizes and estimated the adjusted summary effect size using a 3-parameter selection model using the R package metafor . 36
Finally, we augmented our 3-level modeling framework to evaluate the impact of measurement modality (behavior, neural), psychological construct (identity, emotion), recognition demand (perception, discrimination, recognition), sample age, sample IQ, sex distribution, and study quality assessment scores on FIE differences between autistic and neurotypical samples (see eMethods in Supplement 1 for full operationalization of these moderator variables).
We conducted a series of sensitivity analyses related to prior distribution selection, correlation among upright and inverted conditions, and potential outlier/influential effect sizes. Bayesian inference can be sensitive to prior distribution selection; to test the robustness of our reported results, we compared all model results using a range of various generic prior distributions (eMethods in Supplement 1 ). Since the correlation between upright and inverted conditions was assumed to be moderate ( r = 0.5), we compared all model results across a possible range of values (ie, r = 0.1, r = 0.3, r = 0.5, r = 0.7, r = 0.9). We conducted a leave-1-out analysis, fitting multiple meta-analyses, each excluding a single unique effect size to evaluate the robustness of our results to any single, potentially influential effect size. Finally, we also identified and evaluated the influence of outliers and influential cases for study-averaged effect sizes using dmetar . 39
The full study identification, screening, and selection process is displayed in Figure 1 . Overall, we included 122 effect sizes from 38 studies representing data from 1764 unique individual participants (899 individuals with ASD and 865 neurotypical individuals). Each study included a mean (SD) of 3.21 (3.01) effect sizes, and the mean (SD) study sample age was 18.24 (3.91) years. Overall, the studies included a mean (SD) of 82.6% (12.5%) males and had a mean (SD) of 23.66 (32.19) autistic and 22.76 (18.35) neurotypical individuals. The individual study characteristics, including sample-related and methodological-related information, are displayed in Table 1 . 40 - 64
As expected, we found that neurotypical individuals showed strong evidence of an FIE (upright > inverted) across all studies ( g = 0.95; SE = 0.17; 95% CrI, 0.63-1.30; 100% PPD upright > inverted). In contrast, we did not find strong evidence that autistic individuals showed the same advantage for upright compared with inverted face recognition ( g = 0.52; SE = 0.11; 95% CrI, 0.31 to 0.76; 100% PPD upright > inverted). When comparing the FIE magnitude in ASD and neurotypical groups, we found strong evidence that the FIE was attenuated in ASD compared with neurotypical individuals ( g = −0.41; SE = 0.11; 95% CrI, −0.63 to −0.18); 100% of the PPD was consistent with this interpretation (FIE for individuals with ASD < FIE for neurotypical individuals). As shown in Figure 2 , there was a high degree of heterogeneity among effect sizes ( I 2 = 85.71%), including within-study variability ( I 2 = 25.5%) and moderate between-study variability ( I 2 = 60.21%). We evaluated if heterogeneity in findings could be systematically explained by potential moderators.
We found moderate evidence that the difference in the FIE between autistic and neurotypical samples was moderated by measurement modality ( b = 0.23; SE = 0.24; 95% CrI, −0.25 to 0.7; 83% PPD behavior < N170), psychological construct ( b = 0.46; SE = 0.26; 95% CrI, −0.08 to 0.95; 96% PDD emotion < identity), and recognition demands ( b = 0.37; SE = 0.27; 95% CrI, −0.16 to 0.91; 92% PPD discrimination < perception; Table 2 ).
The difference in the behavioral FIE between autistic and neurotypical samples ( g = −0.51; SE = 0.14; 95% CrI, −0.8 to −0.2) was more negative than the difference in the N170 FIE ( g = −0.28; SE = 0.19; 95% CrI, −0.68 to 0.12; Figure 3 ), suggesting an attenuated FIE in autistic compared with neurotypical samples during later stages of face processing (eg, explicit recognition). Similarly, the difference in the FIE for emotion recognition between autistic and neurotypical samples ( g = −0.79; SE = 0.22; 95% CrI, −1.22 to −0.29) was significantly more negative than the identity recognition FIE difference ( g = −0.33; SE = 0.13; 95% CrI, −0.58 to −0.06), suggesting that the face processing system in autism is less sensitive to inversion when recognizing emotional (compared with identity) information. Finally, the FIE in autism (compared with neurotypical) was reduced during face discrimination ( g = −0.64; SE = 0.2; 95% CrI, −1.08 to −0.25) compared with face perception task paradigm ( g = −0.28; SE = 0.17; 95% CrI, −0.64 to 0.07), suggesting that the magnitude of the FIE in autism may attenuate as cognitive load and/or recognition processes increase (eg, memory demands, encoding and recognition mechanisms).
Differences in the FIE between autistic and neurotypical individuals were not associated with the average sample age ( b = −0.02; SE = 0.01; 95% CrI, −0.05 to 0.01; 96% PPD b = 0) or percentage of males in the sample ( b = −0.16; SE = 0.64; 95% CrI, −1.4 to 1.1; 60% PPD b < 0). The reduced FIE in autism was consistent across all sample ages and sex distributions.
Quality assessment (QA) scores, as measured by the modified Newcastle-Ottawa Scale, ranged between low (QA ≤ 4; k = 12), moderate (QA = 5-8; k = 16), and high (QA ≥ 8; k = 10) ( Table 1 and eResults in Supplement 1 ). We did not find strong evidence that QA scores were systematically associated with FIE differences between autistic and neurotypical groups ( b = 0.04; SE = 0.05; 95% CrI, −0.05 to 0.15; 80% PPD b > 0).
Through visualization and the modified Egger regression test, we found potential evidence of small-study effects based on funnel plot asymmetry ( b = 1.53; SE = 0.26; 95% CrI, 1.04-2.06; 100% PPD intercept > 0; eResults in Supplement 1 ). We further evaluated sensitivity of the primary findings to potential outliers, influential cases, and publication bias. We found 6 studies classified as outliers and/or influential cases (ie, fall outside the 95% CI of the summary effect size or disproportionately contribute to heterogeneity). When excluding these studies, the magnitude of the FIE remained similar in magnitude ( g = −0.45; SE = 0.09; 95% CI, −0.64 to −0.28; P < .001) but substantially reduced heterogeneity across effect sizes ( I 2 = 44.94%). Finally, using a 3-parameter selection model, we did not find significant evidence of publication bias (χ 2 1 = 1.12; P = .29) and the adjusted summary effect size remained similar to the unadjusted estimate ( g = −0.44; SE = 0.10; 95% CI, −0.64 to −0.24; P < .001).
Our leave-1-out analysis showed that these results were robust to any potential outliers and influential effect sizes; 100% of derived summary estimates were between −0.44 and −0.38 (eResults in Supplement 1 ). Furthermore, the reduced FIE in autism was not substantially impacted by the inputted correlation between upright and inverted conditions (0.1: r = −0.4; 0.3: r = −0.41; 0.5: r = −0.41; 0.7: r = −0.41; 0.9: r = −0.41). Finally, the derived results from both the main effect and moderator models were not substantially altered based on prior distribution selection (see eResults in Supplement 1 ).
This systematic review and bayesian meta-analysis investigated sensitivity of the face processing system in autism by evaluating one of the most well-characterized and robust markers of specialized face processing ever studied: the FIE. The FIE has long been characterized as an indirect measure of face specialization, with inverted faces being more difficult to recognize since inversion disrupts the canonical configuration of facial features to which face processing mechanisms are attuned. For the past 30 years, researchers have hypothesized that the face processing system of autistic individuals may differ from that of neurotypical individuals; specifically, reduced use of configural or holistic processing may reduce efficient and accurate recognition of faces, one of the hallmark characteristics of autism. 65 , 66 Here, we determined the veracity of this hypothesis by synthesizing data across the extant literature to determine whether autism is characterized by a reduced FIE and if so, what factors influence the magnitude of this FIE.
These meta-analytic results show that autistic individuals have a moderately attenuated FIE compared with neurotypical peers. Specifically, the inversion cost (upright − inverted) on face recognition performance for neurotypical peers was large ( g = 0.95), which corroborates a wealth of previous literature demonstrating that inverted faces incur an inversion cost (ie, decrease in performance) relative to upright faces. 6 , 8 , 67 In contrary, autistic participants did not show the same meaningful difference in performance for upright compared with inverted faces ( g = 0.52). These findings reconcile the discrepant literature and demonstrate that autistic individuals, on average, show a reduced FIE, suggesting that the face processing systems of autistic individuals are less sensitive to face inversion than those of their neurotypical peers.
An attenuated FIE in autism suggests a face processing system that is less attuned to configural information. Therefore, this implies reduced reliance on configural (as opposed to featural) processing in autism. Configural information refers to the spatial relations of facial features (eg, eyes above nose, nose above mouth, distance between eyes), whereas featural information refers to the features themselves (eg, eyes, nose, mouth). 7 Configural processing facilitates the rapid and efficient extraction of nonverbal facial cues that are rich in social information, 68 including others’ emotional and mental states, which are of themselves complex and dynamic. 69 Developmentally, sensitivity to these nonverbal cues is precursoru to higher-order social cognition, including mentalizing and theory of mind, both of which are critical in successful social interactions. 70 Therefore, reduced sensitivity to configural information in autism may contribute to common clinical features of the condition, including difficulty interpreting nonverbal cues from others and challenges developing and maintaining social interactions and relationships. 71
The FIE was significantly attenuated in autistic compared with neurotypical individuals when assessed with electrophysiological (eg, N170 amplitude and latency) and behavioral measures of face recognition (eg, accuracy, % correct, error rate). However, the behavioral FIE was more attenuated than the N170 FIE. The behavioral FIE is defined as the reduced accuracy in recognizing inverted (compared with upright) faces, 8 whereas the N170 FIE represents increased N170 amplitude and latency to inverted (compared with upright) faces. 11 , 72 These measures reflect different stages of processing (early and late face processing) with considerable differences in task demands (cognitive load, encoding, and recognition phases). Given that the N170 represents structural encoding of faces, the more attenuated FIE in autism at subsequent perceptual stages (ie, behavior) may reflect less specialization at structural encoding that compounds throughout later stages of face processing.
The FIE was significantly more attenuated in autistic compared with neurotypical individuals during emotion recognition compared with identity recognition of faces. Although the FIE has historically been specific to face identity recognition, inversion effects are hypothesized to be even larger for emotion recognition. 73 , 74 Additionally, interpreting emotions from faces, not identity, is part of the diagnostic criteria for autism, 71 suggesting sensitivity to emotion processing is more characteristic of the clinical phenotype. These results provide indirect support of reduced specialization in the emotional face processing system in autism, which may contribute to frequently occurring clinical features and may be a downstream consequence of reduced attention to faces during childhood. 5 , 75
Future studies should evaluate the FIE in autism in relation to nonface objects to help disentangle the specificity of the configural processing mechanism to faces. Additionally, longitudinal studies, which directly link inversion effects to downstream development of social cognitive processing (ie, mentalizing and theory of mind) will confirm long-standing hypotheses in autism face perception research.
Few studies included adolescent and emerging adult participants or an adequate number of females to make claims about age- and sex-related differences in the FIE, thereby limiting the generalizability of these findings. Also, most included studies (30 of 38) did not include a nonface control condition, which limited our ability to understand the face specificity or disproportionality of the FIE in autism. Finally, 12 articles met inclusion criteria but did not report data required for synthesis. Following email request for data, only 2 of 10 authors provided such data, which limited the potential precision of this study. Finally, this study was not preregistered, thereby increasing the potential risk of bias.
Based on this comprehensive meta-analysis, autism was associated with a reduced FIE, suggesting that autistic individuals may have less perceptual face expertise or a less specialized face processing system in comparison to neurotypical peers. This was particularly true for emotion (compared with identity) recognition, which may underlie common clinical symptomology in autism (eg, differences in interpreting emotional expressions).
Accepted for Publication: May 3, 2023.
Published Online: July 5, 2023. doi:10.1001/jamapsychiatry.2023.2105
Corresponding Author: James C. McPartland, PhD, Yale Child Study Center, Yale University School of Medicine, New Haven, CT 06510 ( [email protected] ).
Author Contributions: Drs Griffin and McPartland had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Griffin, McPartland.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Griffin.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Griffin.
Obtained funding: McPartland.
Administrative, technical, or material support: Griffin, Keifer, McPartland.
Supervision: Griffin, McPartland.
Other - data quality control: Azu, Cramer-Benjamin, Franke, Herman, Iqbal, Rosenthal.
Conflict of Interest Disclosures: Dr McPartland reported stock options from Modern Clinics; serves on the scientific advisory boards of Modern Clinics and Pastorus; royalties from other from Guilford, Lambert, Oxford, and Springer; consulting for BridgeBio, Customer Value Partners, Determined Health, and BlackThorn Therapeutics; and research funding from Janssen Research and Development outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by the Hilibrand Foundation and the National Institute of Mental Health (grant U19 MH108206)
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Information: This meta-analysis was not preregistered. All materials, including data, analysis, and visualization code, have been made publicly available ( https://osf.io/5kxd4/?view_only=55bdcd0262af4d99a247557098539bb3 ).
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

- Advanced Search
Identification of autism spectrum disorder based on electroencephalography: : A systematic review
New citation alert added.
This alert has been successfully added and will be sent to:
You will be notified whenever a record that you have chosen has been cited.
To manage your alert preferences, click on the button below.
New Citation Alert!
Please log in to your account
Information & Contributors
Bibliometrics & citations, view options, recommendations, robot-assisted autism spectrum disorder diagnostic based on artificial reasoning.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that affects people from birth, whose symptoms are found in the early developmental period. The ASD diagnosis is usually performed through several sessions of behavioral observation, ...
An inclusive design approach for developing video games for children with Autism Spectrum Disorder
The efficacy of therapeutic treatments for Autism Spectrum Disorder is mainly associated with the treatment's intensity in terms of weekly hours. This has led mental health professionals to explore the use of video games to complement traditional ...
Exploiting Augmented Reality in LEGO Therapy for Children with Autism Spectrum Disorder
Numerous computer-based therapies have been designed for cognitive-behavioral interventions to support children with Autism Spectrum Disorder (ASD) in recent years. Among these technologies, Augmented Reality (AR) offers unique educational ...
Information
Published in.
Pergamon Press, Inc.
United States
Publication History
Author tags.
- Autism spectrum disorder
- Electroencephalography
- Machine learning
- Deep leaning
- Multi-model fusion
- Review-article
Contributors
Other metrics, bibliometrics, article metrics.
- 0 Total Citations
- 0 Total Downloads
- Downloads (Last 12 months) 0
- Downloads (Last 6 weeks) 0
View options
Login options.
Check if you have access through your login credentials or your institution to get full access on this article.
Full Access
Share this publication link.
Copying failed.
Share on social media
Affiliations, export citations.
- Please download or close your previous search result export first before starting a new bulk export. Preview is not available. By clicking download, a status dialog will open to start the export process. The process may take a few minutes but once it finishes a file will be downloadable from your browser. You may continue to browse the DL while the export process is in progress. Download
- Download citation
- Copy citation
We are preparing your search results for download ...
We will inform you here when the file is ready.
Your file of search results citations is now ready.
Your search export query has expired. Please try again.

Introduction
Reading in autism spectrum disorders: a literature review.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Fernanda Dreux Miranda Fernandes , Cibelle Albuquerque de La Higuera Amato , Carla Cardoso , Ana Luiza Gomes Pinto Navas , Daniela Regina Molini-Avejonas; Reading in Autism Spectrum Disorders: A Literature Review. Folia Phoniatr Logop 1 March 2016; 67 (4): 169–177. https://doi.org/10.1159/000442086
Download citation file:
- Ris (Zotero)
- Reference Manager
Objective: To review what the literature says about reading abilities of children on the autism spectrum (autism spectrum disorders, ASD) as well as to assess the results of intervention proposals. The broad ASD diagnosis used in the last decades and the resulting changes in the prevalence of these disorders have led to a relevant increase in the number of children diagnosed with ASD in the school system. The purpose of this review is to identify the different profiles of reading abilities shown by children with ASD described in the recent literature and the results of reported intervention methods. Methods: A review of the literature was conducted in the Web of Sciences and PubMed databases with the keywords ‘autism' AND ‘read*' and the filter 2010-2015. All articles published in the last 5 years focusing on description of and intervention for reading abilities in individuals with ASD were included. Review articles were excluded. Results: The selected 58 articles were divided into those that described reading abilities in individuals with ASD (n = 27) and those that reported intervention procedures for reading development (n = 31). Conclusions: Direct comparisons and associations were prevented due to different inclusion criteria and lack of detailed information about intervention processes. We propose tentative conclusions that should be confirmed by further studies.
The increasing number of children with a diagnosis of autism spectrum disorders (ASD) in the school system demands consistent information about the characterization of their reading abilities and the results of different intervention alternatives.
ASD have been the focus of many studies based on several different perspectives. These may vary from genetic and neurologic correlates [ 1,2,3,4 ] to social and emotional impact [ 5,6,7 ], or educational issues [ 8,9 ], family perspectives [ 10,11 ] and different intervention proposals [ 12,13 ].
The definitions and diagnostic criteria for ASD vary significantly in different studies [ 14,15 ], and therefore the conclusions can hardly be compared or accumulated, providing consistent data. The changes in the definition of what should be included within the autism spectrum are just one of the many variations that must be considered [ 16,17 ].
The changes implemented in the DSM-5 classification criteria will probably lead to different groups of individuals receiving the diagnosis of ASD [ 18,19 ]. Therefore, comparing the results of studies conducted before and after these changes may become even more complicated. It can be assumed that the vast majority of the subjects in studies published until 2015 were diagnosed according to the DSM-IV criteria. However, it is virtually impossible to determine a time frame from which all papers refer to subjects diagnosed according to the DSM-5 criteria.
On the other hand, the broad ASD diagnosis used in the last three decades and the resulting changes in the prevalence of these disorders [ 20,21 ] have led to a relevant increase in the number of children diagnosed with ASD in the school system [ 22 ].
Regarding reading abilities, studies should describe whether they refer to decoding, such as performance in tasks of word recognition performance, or in a broader sense to word reading comprehension [ 23 ]. Children with ASD are often characterized as showing precocious word reading abilities [ 24 ], but even though these children may have good decoding skills, comprehension is impaired in most cases.
Considering these aspects, it is relevant to know, at this point, what the recent literature describes about reading abilities in children with ASD and the intervention approaches proposed to improve such skills. Therefore, a literature review was performed with the purpose to address the questions: ‘do children with autism have specific reading impairments?' and ‘do interventions with focus on reading abilities of children with autism have positive results?'.
The purpose of this review was to identify the different profiles of reading abilities of children with ASD and the results of different intervention methods reported in the literature.
Search Strategy
A review of the literature was conducted to answer the questions stated above. The Web of Sciences (WoS) and PubMed (PM) databases were searched with the keywords ‘autism' AND ‘read*' with the filter 2010-2015.
Inclusion criteria were: articles published in the last 5 years in peer-reviewed journals indexed in WoS and PM databases with the focus on a description of reading abilities and intervention with individuals with ASD. Review articles were excluded.
In order to obtain an overview of the available information about the characterization of reading abilities and intervention proposals no further criteria were applied in the selection of the reviewed papers.
The first search resulted in 782 articles; 604 in WoS and 178 in PM. The initial analysis aimed to determine which of them focused specifically on both autism and reading. This process resulted in 58 papers in WoS and 32 in PM, a total of 90 articles. They were further analyzed to eliminate duplicates (i.e. articles that were included both in WoS and PM) and publications that did not have enough data; this search resulted in 72 articles that were analyzed according to their content. Among these, 14 articles were reports on literature reviews and were, therefore, excluded. The remaining 58 were divided into two categories: (a) those that described the reading abilities of individuals with ASD (n = 27) and (b) those that reported intervention procedures towards reading development (n = 31) (fig. 1 ). Further inspection of the articles helped specifying the details of each study, number of participants, measures of literacy skills used, and main conclusions. The results are presented in tables 1 and 2 .
Articles about reading characteristics of persons with ASD
Articles describing intervention proposals regarding reading abilities of persons with ASD

Search and selection process.
It can be observed that the number of participants in each study varied significantly. There are several papers describing studies with a relatively large numbers (59% of them report studies with more than 20 participants), some single-case studies (7%), and 4 studies that did not report the number of subjects (14%). Only 11% of 27 articles described studies with adults.
Regarding the type of measures used to evaluate literacy skills, most studies (44%) focused on single-word reading and text comprehension measures, some (29%) assessed cognitive abilities that are related to reading such as memory and oral language skills, whereas only 2 studies investigated spelling skills in this specific population.
Although the inclusion criteria for the participants in each study are not equivalent across the different papers, thus preventing a true meta-analysis, some tentative conclusions can be drawn.
- Performance in single-word reading tasks is better than reading comprehension.
- The oral language level is associated with reading comprehension.
- Persons with ASD have difficulties with reading comprehension despite eventually good or intact decoding skills.
- There is no confirmation of the weak central coherence hypothesis; it seems that the lack of association between meaning and word recognition is based on other factors.
- Nonverbal social and cognitive abilities are associated with reading comprehension and reading performance.
- Phonological processing seems to be less associated with reading comprehension performance than semantics and syntactic knowledge.
Contrary to what could be observed in articles describing reading abilities of persons with ASD, most of the papers describing intervention procedures had a small number of subjects [22 papers (70%) had 6 participants or less]. In total, these papers reported on 62 children and 5 adults. Six articles reported interventions with more than 18 participants, leading to a total of 143 children in these larger-number studies.
The intervention procedures described can be divided in two groups: those aiming to improve single-word reading and those directed to reading comprehension. Behavioral techniques are the basis for the strategies used in studies that focused on improving single-word reading. The conclusions of these articles imply that not all progress was generalized or maintained after the end of the training programs. The papers reporting programs for enhancing reading comprehension describe different approaches such as computer-assisted instruction, direct instruction, talking about a book, graphic organizers, story maps and prompting. Generalization to other abilities and stability of improvement were reported by these studies.
Three other articles refer to suggestions of intervention strategies such as scaffolding and the use of software designed to improve reading comprehension. These approaches are described as flexible, allowing one-to-one adaptations.
Characteristics of the reading process of persons with ASD were described in 27 articles that included 1,490 individuals. Direct comparisons and associations are prevented by the different inclusion criteria used in the different studies. However, it is possible to propose some preliminary conclusions that should be confirmed by further studies. Reading comprehension seems to be more associated with semantic and syntactic abilities in oral language than with phonological development. Decoding skills, apparently, are not directly associated with reading comprehension, thus leading to better performance in single-word reading tests than in reading comprehension. Nonverbal social and cognitive abilities seem to be associated with reading comprehension and reading performance, especially in what refers to the association of meaning to a word.
Intervention proposals regarding reading abilities of persons with ASD are reported in 31 articles that refer to a total of 210 subjects, including just 5 adults. Not all studies provide the detailed descriptions of the intervention procedures that would be necessary to make comparisons and associations between them. Very few studies include information about the duration of the intervention and the prior training of the therapists. Therefore, any conclusion about the reasons for the reported results would be premature. Better and more stable results are described by the articles that report interventions focused towards the improvement of reading comprehension, as opposed to the studies regarding the use of behavioral techniques to increase single-word reading abilities. However, there is not enough data about the duration of the intervention processes, specific characteristics of the participants before the intervention, training of the therapists in the area, specific material or resources used to allow hypotheses about better or more successful intervention methods.
Literacy acquisition in children and adults with ASD demand further assessment. The large individual variations of the autism spectrum may be reflected in the reading performance of persons with ASD, therefore resulting either in hyperlexia or in different forms of reading deficits. The identification of different reading strategies and specific profiles of abilities and impairments depends on efficient assessment tools that are essential to the design of more efficient intervention procedures.
Email alerts
Citing articles via, suggested reading.
- Online ISSN 1421-9972
- Print ISSN 1021-7762
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(12); 2023 Dec
- PMC10771115

Exploring Early Childhood Autism Spectrum Disorders: A Comprehensive Review of Diagnostic Approaches in Young Children
Ruba a alrehaili.
1 Family Medicine, Academy of Family Medicine, Ministry of Health, Al-Madinah, SAU
Reem M ElKady
2 Radiology and Medical Imaging, Faculty of Medicine, Taibah University, Al-Madinah, SAU
Jumana A Alrehaili
3 College of Medicine, Taibah University, Al-Madinah, SAU
Reem M Alreefi
Autism spectrum disorders (ASD) encompass a diverse range of developmental disabilities characterized by pervasive deficiencies in socialization, communication, and the manifestation of behavioral issues. This review aims to clarify the diagnostic criteria for ASD, explore available diagnostic tools, evaluate biomedical examinations facilitating ASD diagnosis, and discuss the approach to screening assessments for ASD. ASDs are prevalent conditions, with a globally estimated prevalence of 7.6 cases per 100 (one in 132), based on a comprehensive review of epidemiological studies. The precise cause of autism and other ASDs remains unknown; however, genetic and environmental factors likely contribute. Common signs and symptoms shared among individuals with autism include restricted repetitive behaviors and learning disabilities. Standardized methods, such as the Diagnostic and Statistical Manual, Fifth Edition (DSM-5), diagnostic instruments, a thorough medical history, multiple physical examinations, laboratory investigations, neuroimaging, and screening tests, play a pivotal role in early autism diagnosis and intervention. ASD is a lifelong neurodevelopmental condition characterized by multiple deficits. Early detection is anticipated to have a positive impact on outcomes.
Introduction and background
Autism spectrum disorders (ASD) constitute a complex neurodevelopmental disorder influenced by a spectrum of genetic, pathophysiological, and environmental factors, typically manifesting early in childhood and persisting throughout life [ 1 ]. The prevalence of ASD among school-aged children has surged from one in 150 in 2000 to one in 54 in 2016, highlighting its relatively common occurrence [ 2 ].
ASD represents a cluster of developmental disabilities characterized by deficits in social communication and the presence of restricted or repetitive interests and behaviors, aligning with the diagnostic criteria outlined in the Diagnostic and Statistical Manual, Fifth Edition (DSM-5) [ 3 ]. Research has consistently shown that children diagnosed with ASD often exhibit behavioral problems, including hyperactivity, poor attention, impulsivity, aggression, self-injury, and challenges in understanding social cues such as body language, gestures, and facial expressions. Moreover, these children frequently display unusual responses to sensory stimuli, such as hypersensitivities to light, sound, color, smell, or touch, and may demonstrate a heightened pain threshold [ 4 ].
Additionally, children with ASD manifest qualitative abnormalities in motor performance, which, although not part of the primary diagnostic criteria are believed to contribute to their clinical presentation and overall challenges [ 5 , 6 ]. Typically, children with ASD require substantial levels of supervised care, specialized educational resources, and healthcare services [ 7 ].
The diagnosis of ASD usually takes place in early childhood through behavioral observations, clinical presentations, or various imaging techniques such as functional MRI and diffusion tensor imaging. Imaging techniques often reveal abnormalities in volume associated with structural connectivity and atypical functionality in the brain. Magnetic resonance spectroscopy (MRS), a method allowing the quantification of various brain metabolites, including N-acetyl-aspartate (NAA), has reported a significant decrease in NAA concentrations in patients with ASD [ 8 ].
ASD necessitates a comprehensive, systematic, and structured approach that extends beyond the mere identification of the disorder. The diagnostic assessment provides invaluable insights into the child's strengths and weaknesses, laying the foundation for a well-informed roadmap for treatment [ 9 ]. The significance of an early and reliable ASD diagnosis cannot be overstated, as prompt interventions are essential once signs manifest.
ASD is a complex neurodevelopmental disorder characterized by three primary features: speech delay, challenges in social interaction and communication, and the presence of repetitive behaviors or desires [ 10 ]. Beyond these primary symptoms, autistic children may exhibit comorbidities such as learning disabilities, neurological conditions, depression, digestive deficiencies, and insomnia. In 1943, Kanner introduced the term "autism" for the first time [ 11 ]. Over the years, the diagnostic framework has undergone expansion to encompass various manifestations of autism. ASD includes autism, Asperger's disorder, autistic disorder, childhood disintegrative disorder, adolescent autism, and general developmental disorder not otherwise specified.
Epidemiology
The prevalence of ASD varies globally, ranging from 0.02% to 3.66% [ 12 ]. There is a higher frequency observed in males than females [ 13 ].
In different continents, the prevalence of ASD differs, with rates of 0.4% in Asia, 1% in America, 0.5% in Europe, 1% in Africa, and 1.7% in Australia [ 12 ]. The Gulf Cooperation Council (GCC) countries show an increasing prevalence of ASD, considering it one of the most common impairments [ 14 ]. Specific rates include 29/10,000 in the UAE, 1.4/10,000 in Oman, and 4.3/10,000 in Bahrain [ 14 ]. In 2002, Saudi Arabia identified 42,500 cases of autism, along with additional cases that went undiagnosed [ 15 ].
A recent systematic review disclosed that the prevalence of ASD in Arabian Gulf countries, including Saudi Arabia, ranged from 1.4 to 29 cases per 10,000 population, notably lower than the rates reported in developed countries (39 to 77 cases per 10,000) due to underdiagnosis [ 16 ]. A cross-sectional study involving 205 individuals diagnosed with ASD revealed a male-to-female ratio of 4.9:1. Comorbidities were present in 65% of patients, with attention-deficit hyperactivity disorder (ADHD) being the most prevalent (53%), followed by intellectual disability (8%), epilepsy (2%), and cerebral palsy (2%) [ 17 ].
The specific cause of ASD remains uncertain, and hypotheses about its origins have evolved over time. While a psychosocial hypothesis linking autism to defective childbearing was once considered, it has been firmly rejected. Current evidence strongly supports the idea that the etiology of ASD is multifactorial, primarily rooted in genetics [ 18 , 19 ].
Family studies emphasize the familial and heritable nature of autism, with siblings of autistic children having a recurrence ratio ranging from 2% to 8%, surpassing that of the general population [ 20 , 21 ]. Twin studies reveal a higher concordance rate in monozygotic twins (90%) compared to dizygotic twins (10%) [ 21 , 22 ]. Previous research has explored the genetic underpinnings of autism through the identification of gene mutations or copy number variations at specific chromosomal locations associated with neurodevelopmental processes in individuals and families [ 23 , 24 ]. Noteworthy genes linked to the causation of autism, such as FOXP2 , RAY1/ST7 , IMMP2L , and RELN at 7q22-q33, have been recognized [ 25 ]. A study conducted by the International Molecular Genetic Study of Autism Consortium, encompassing 99 multiplex families, identified regions related to ASD on six different chromosomes, with chromosome 7 holding particular significance [ 26 ].
Various environmental factors are recognized as potential contributors to autism. Epidemiological studies point to prenatal viral infections, including measles, mumps, rubella, chicken pox, varicella-zoster, and cytomegalovirus infections, as contributing factors [ 27 ]. Cytomegalovirus, particularly, has been identified as a causative agent leading to enduring neurological impairment, affecting around 10%-20% of newborns when the mother experiences infection during pregnancy [ 27 ].
The correlation between maternal age, paternal age, and autism risk has been identified as a significant factor in several studies [ 28 , 29 ]. Sandin et al., in a meta-analysis, found a lower risk for autism (relative risk of 0.76) for mothers under 20 compared to those aged 25-29. Conversely, mothers aged 35 or older had a higher relative risk (1.52) compared to mothers aged 25-29 [ 30 ]. Reichenberg et al., in a study based on the population, indicated that the likelihood of autism starts to increase at the age of 30 and continues to rise after reaching the age of 50 [ 29 ]. Furthermore, zinc deficiency has been implicated in certain cases, where prolonged deficiency during pregnancy may result in various dysfunctions in embryonic growth, particularly in neurodevelopment [ 31 ]. Initial exploration into the relationship between zinc and autism originated from its association with neurodegeneration and dysfunction [ 32 ]. Recent studies also propose that the increased risk of ASD is associated with both the uptake of toxic metals and deficiencies in essential elements [ 33 ]. Notably, zinc has been observed to interact with β-amyloid and its precursors, which are crucial factors in the brain's degenerative processes [ 32 ].
Symptoms and signs of ASD
Social Communication
The early symptoms of ASD often manifest as delays and difficulties in social interaction, yet these signs may be subtle and easily overlooked. The absence of mutual attention, characterized by a failure to demonstrate interest and share a focus of attention, is particularly indicative of ASD [ 34 ]. Caregivers should be attentive to the child's consistent lack of response to their name, as this may suggest potential hearing impairment or ASD.
Additionally, stereotypical behaviors, such as insufficient facial expressions, the lack of a social smile, and limited use of gestures (e.g., clapping, smiling, shaking the head, nodding), are frequently observed. Children with ASD may exhibit a diminished understanding of the emotions of others and may be less attuned to the impact of their own attitudes on those around them [ 35 ].
Restricted, Repetitive Behaviors, Interests, and Activities
Restricted, repetitive behaviors, interests, and activities are commonly observed in children with ASD. These behaviors may include motor attitudes such as twirling, finger flicking, hand flapping, and headbanging [ 34 ]. It is widely acknowledged that children with ASD often engage in the repetitive use of items, such as lining up toys, and the repetitive use of words, specifically stereometric words with constant shape or pattern, as well as restricted echolalia. The term "restricted echolalia" refers to the specific imitation or reproduction of speech, that is repeated shortly after it has been heard [ 35 ].
Learning Disabilities
Historically, individuals with severe learning disabilities (defined as an IQ less than 70) were predominantly identified as having autism. Within the spectrum continuum, comorbid learning disorders are estimated to affect nearly 50% of individuals with ASD. These individuals may exhibit an irregular cognitive profile, characterized by significant variations between verbal and non-verbal scores in either direction. It is crucial to note, however, that individuals with "higher" scores in either verbal or non-verbal abilities may not necessarily reflect enhanced social skills or proficiency in daily coping life skills, which are likely to be severely affected [ 35 ].
Diagnostic criteria for ASD
Diagnostic criteria and severity level are shown in Table Table1 1 [ 36 ].
ASD, autism spectrum disorder.
| Criteria | Symptoms | Severity Level for ASD |
| A. Persistent Deficits in Social Communication and Social Interaction | ||
| 1. Deficits in relationships | Challenges in adapting behavior to different social contexts, difficulties in engaging in imaginative play or forming friendships, lack of interest in interpersonal connections | Level 3: Requiring very substantial support |
| 2. Deficits in social-emotional reciprocity | Abnormal social approaches, failure of normal back-and-forth conversation, reduced sharing of interests, emotions, or affect, inability to initiate or respond to social interactions | Level 2: Requiring substantial support |
| 3. Deficits in nonverbal communicative behaviors | Poorly integrated verbal and nonverbal communication, abnormalities in eye contact and body language, deficits in understanding and using gestures, loss of facial expressions and nonverbal communication | Level 1: Requiring support |
| B. Restricted, Repetitive Patterns of Behavior, Interests, or Activities | ||
| 1. Insistence on sameness | Inflexible adherence to routines or ritualized patterns of behavior, extreme distress at minor changes, difficulties with transitions, rigid thinking patterns, greeting rituals, or a need for routine | Level 3: Requiring very substantial support |
| 2. Stereotyped or repetitive behaviors | Simple motor stereotypes like lining up toys or flipping objects, engaging in echolalia and using idiosyncratic phrases, repetitive motor movements, use of objects, or speech | Level 2: Requiring substantial support |
| 3. Hyper- or hyporeactivity to sensory input | Apparent indifference to pain or temperature, adverse responses to specific sounds or textures, excessive smelling or touching of objects, visual fascination with lights or movement | Level 1: Requiring support |
| 4. Highly restricted and fixated interests | Abnormal intensity or focus, strong attachment to or preoccupation with unusual objects, excessively circumscribed or perseverative interests | Level 1: Requiring support |
| C. Symptoms in Early Childhood | ||
| 1. Early caregiver reports | No longer deemed essential | |
| 2. Early childhood | Approximately age 8 and younger | |
| D. Symptoms Limit and Impair Everyday Functioning | ||
| 1. Minimal rituals and repetitive behaviors (RRBs) impairments | Significant difficulties with functioning due to RRBs, resistance to efforts to disrupt or divert RRBs from fixed interests | Social communication severity: Level 3, restricted interests severity: level 3 |
| 2. Minimal social impairment | Social communication deficiencies result in noticeable impairments, challenges in facilitating social encounters, and atypical or ineffective reactions to other people's social overtures | Social communication severity: level 1, restricted interests severity: level 1 |
| E. Disturbances Not Better Explained | ||
| Not better explained by intellectual disability or global developmental delay | Intellectual impairment and ASD may co-occur, and social communication may persist below the predicted average developmental level in comorbid cases |
Diagnostic instruments for ASD
Evaluation of a Child With ASD
The primary objective of the ASD evaluation is to identify effective strategies and care tailored to the child and family. This involves recognizing coexisting conditions and associated developmental issues that could negatively impact the patients and their families [ 37 ].
Diagnosing ASD can be challenging; the National Institute for Health and Care Excellence (NICE) offers recommendations on the assessment and treatment of ASD, urging clinicians in the United Kingdom to guarantee that children and adolescents under suspicion of ASD can avail the services of a regional multidisciplinary autism team. This team assumes a pivotal role in offering support and specialized knowledge in the evaluation and creation of profiles for individuals and their families [ 34 ].
ASD is identified through a thorough examination of developmental history and behavioral observation across settings like school, home, and social care. Documentation should encompass behavior duration, severity, and consequences. Despite consistent characteristics, their expression may vary in different environments. The analysis should consider the child's overall development, medical history, and potential risk factors [ 38 ].
Importantly, the absence of other symptoms and signs, such as poor eye contact or social smiling, does not rule out an ASD diagnosis. It is essential to acknowledge that the absence of parental apprehension regarding early development does not automatically imply a standard developmental trajectory [ 39 ].
Examination
As part of the evaluation, a comprehensive physical examination is necessary, incorporating a thorough neurological assessment, dysmorphism regulation, scrutiny for neurocutaneous stigma, and a Woods light (ultraviolet light) examination [ 40 ]. It is imperative to identify signs of illness, self-harm, and potential ill-treatment during this process.
Laboratory Investigations
ASD is recognized as a developmental neurological disorder, prompting numerous studies to identify potential biomarkers associated with its development.
Elevated whole-blood serotonin, or hyperserotonemia, represents the first identified biomarker in ASD, present in over 25% of affected children [ 41 ]. The contribution of the serotonin system to ASD pathophysiology remains incompletely understood, with emerging data from neuroimaging and postmortem samples indicating changes in the brain serotonin system in ASD [ 41 ].
Unusual patterns of visual preference, as gauged by eye-tracking, are considered hallmarks; however, their utility as an early biomarker for ASD remains uncertain [ 42 ].
Melatonin deficit has been reported in several studies based on plasma or urine samples from individuals with ASD [ 43 ].
Identification of small-molecule peptides using blood plasma proteomic profiling and examination of cerebrospinal fluid can reveal modified patterns of proteins/peptides [ 33 ].
Proteomic analysis of serum from children with autism has revealed differential expression of apolipoproteins and other components of protein complements [ 43 ].
Magnetic Resonance Imaging (MRI)
Structural MRI: The literature on structural MRI in ASD provides significant evidence of volume differences in both gray and white matter, revealing distinct region-specific variations [ 44 ]. Examinations of the basal ganglia have revealed an increased size of the caudate in individuals with autism, and this enlargement corresponds with the intensity of repetitive behaviors [ 44 ]. Establishing a connection between neuroanatomical observations and behavioral manifestations is essential for understanding the impact of structural alterations on the origins of ASD.
A noteworthy discovery in the literature is the swift expansion of brain volume in early childhood, characterized by an approximately 10% rise, reaching its peak around 2-4 years [ 44 ]. This initial phase of accelerated growth is usually followed by a stabilization, evident in both gray and white matter. Research suggests a more pronounced association with the growth of white matter in early childhood, as indicated by meta-analyses conducted by Amaral et al. and Stanfield et al., proposing that age plays a crucial role and this type of enlargement is predominantly observed in young children with ASD [ 45 , 46 ].
The diversity of these observations emphasizes that autism affects both gray and white matter. Recent advancements in technology now allow for a more nuanced assessment of the composition of gray and white matter structures. Specifically, cortical gray matter can be precisely characterized in terms of cortical thickness and surface area, providing a collective estimate of cortical gray matter volume. Cortical thickness serves as an indicator of dendritic arborization and pruning within gray matter or alterations in myelination at the white-gray matter interface. Meanwhile, variations in surface area correspond with the extent of cortical folding or gyrification, believed to be influenced by the division of progenitor cells during embryogenesis in the periventricular region [ 44 ]. The analysis of variations in these measurements of cortical gray matter yields crucial insights into early neuroanatomic developmental events in the ASD population.
Functional magnetic resonance imaging (fMRI): fMRI studies uncover distinctive neuroanatomical patterns in ASD. Hyperactivation is observed in subcortical structures, encompassing the bilateral thalamus, bilateral caudate, and the right precuneus, particularly evident during emotional-face processing. In contrast, there is hypoactivation in the hypothalamus during the same emotional-face processing task. Sub-analyses with more homogeneous contrasts confirm and preserve the key findings of the main analysis, emphasizing the hyperactivation in subcortical structures [ 47 ].
Notably, abnormalities in subcortical structures, including the amygdala, hypothalamus, and basal ganglia, are intricately linked to atypical emotional-face processing observed in individuals with ASD [ 47 ]. These findings shed light on the intricate interplay between neuroanatomical structures and emotional processing in the context of ASD, advancing our understanding of the neural underpinnings of this complex developmental disorder.
Screening Recommendations
Studies have demonstrated that ASD can often be identified at or below 18 months of age, with a more accurate evaluation possible by age 2 [ 48 ]. However, conclusive diagnoses may not be reached until later stages, underscoring the potential delay in necessary care for children with ASD. Early diagnosis significantly influences the initiation of effective recovery programs. The American Academy of Pediatrics (AAP) recommends developmental screening at 9, 18, and 30 months during regular well-child visits. Additional screening may be necessary for children at elevated ASD risk or displaying signs, emphasizing the importance of screening all children, particularly those at higher risk due to factors such as preterm birth or having a family history of ASD [ 48 ].
Developmental Screening Tools
Various screening tools assist in identifying children with developmental delays, either disorder-specific or covering multiple areas. The CDC does not endorse specific tools, and the list is non-exhaustive, with options for pediatric practices, school systems, and community settings [ 48 ].
Types of screening tools: Several developmental screening tools exist, and selected examples include the following.
Communication and Symbolic Behavior Scales (CSBS): A standardized instrument assessing communication and symbolic skills up to the 24-month level. The Infant-Toddler Checklist is a screening tool completed by parents.
Ages and Stages Questionnaires (ASQ): A comprehensive developmental screening tool, consisting of a parent-completed questionnaire with sections tailored to specific age groups, screening across various domains.
Modified Checklist for Autism in Toddlers (M-CHAT): Parent-completed questionnaire identifying children at risk for autism in the general population.
Parents’ Evaluation of Developmental Status (PEDS): An overall developmental screening tool. It is a parent interview form designed to screen for developmental and behavioral issues, utilizing a single response form applicable to all age groups [ 49 ].
Behaviors and presentations exhibit temporal variations, showing a tendency for improvement across various domains, though substantial individual variability exists. A majority of individuals necessitate consistent and precise support throughout their lives. While many adults require ongoing full-time assistance, a small percentage of those with higher-functioning ASD (15%) may achieve independence and gain employment [ 43 ].
Critical factors influencing outcomes include the severity of behavioral manifestations, cognitive capacities, and verbal abilities. Researchers have reported promising outcomes for individuals undergoing early interventions that emphasize skill development. However, further research is imperative to ascertain whether these early interventions can exert a lasting impact on individuals with ASD [ 50 ].
Treatments and interventions
ASD therapies encompass a spectrum of medical, clinical, speech/language, behavioral, physical therapy, supportive, and alternative approaches to medication. In the treatment of children within the target age range for routine ASD screening, behavioral interventions, particularly early comprehensive behavioral and developmental treatments, play a predominant role. These interventions may employ strategies that integrate elements of cognitive-behavioral therapy, parent reinforcement components, and play or interaction-based approaches [ 50 ]. Among the various therapeutic approaches, those centered on integrated behavioral analysis present the highest-quality evidence showcasing their impact on cognitive and language outcomes. Implementation of such strategies may occur in a home or school environment, typically demanding a significant time commitment, with certain programs extending up to 40 hours a week [ 51 ].
Applied behavior analysis (ABA) is a treatment firmly rooted in learning theories and behavioral conditioning. It utilizes precise intervention objectives paired with positive reinforcement, including verbal praise, tokens, or rewards [ 52 ]. An essential element of ABA is the repetition of learning trials. A comprehensive meta-analysis assessing the efficacy of ABA interventions for young children diagnosed with autism demonstrated moderate to substantial positive impacts on intellectual functioning, language development, acquisition of daily living skills, and social functioning [ 53 ]. Significantly larger effect sizes were evident in outcomes related to language. Another noteworthy intervention is pivotal response treatment (PRT), which utilizes a more naturalistic behavioral approach focusing on particular skills and motivations, particularly pivotal areas [ 54 ]. In a randomized controlled trial, PRT exhibited advantages for functional and adaptive communication skills in 53 children (aged 2 to 6 years) diagnosed with autism and significant language delay [ 55 ]. In comparison to ABA, PRT was regarded as more effective in enhancing verbal expressive communication and associated with a diminished occurrence of disruptive behaviors.
Approximately 70% of individuals with autism also experience concurrent mental health conditions, including ADHD, irritability, aggression, as well as mood and anxiety issues [ 56 ]. Risperidone and aripiprazole have received FDA approval for addressing irritability associated with ASD, with risperidone approved for children aged at least five years and aripiprazole for those at least six years old [ 56 ]. Prolonged studies assessing the effectiveness of risperidone in ASD have demonstrated its efficacy in reducing behavioral symptoms and impulsivity compared to haloperidol. Additionally, a two-month randomized, double-blind, placebo-controlled trial found no significant differences between aripiprazole and risperidone in children and adolescents with ASD and associated behavioral symptoms. This evaluation considered primary outcome measures (ABC scores) and safety measures, including alterations in appetite and weight gain [ 57 ].
Conclusions
ASD, characterized by behavioral challenges in social interactions and repetitive patterns of behavior, activities, and interests, is a heterogeneous and enduring neurodevelopmental disorder. The exact etiology of ASD remains elusive, yet evidence strongly points toward a significant hereditary influence. The diagnosis of suspected ASD in children involves a prompt and multidisciplinary procedure, with local pathways facilitating connections to specialized ASD teams. Various diagnostic modalities, including lab investigations and neuroimaging, contribute to the comprehensive assessment of ASD. Screening assumes a pivotal role in the early detection of ASD, with the anticipation that early diagnosis and early intervention can positively influence outcomes. Recognizing and effectively addressing associated morbid conditions is imperative for comprehensive care.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Jumana A. Alrehaili, Ruba A. Alrehaili, Reem M. ElKady, Reem M. Alreefi
Acquisition, analysis, or interpretation of data: Jumana A. Alrehaili, Ruba A. Alrehaili, Reem M. ElKady, Reem M. Alreefi
Drafting of the manuscript: Jumana A. Alrehaili, Ruba A. Alrehaili, Reem M. ElKady, Reem M. Alreefi
Critical review of the manuscript for important intellectual content: Jumana A. Alrehaili, Ruba A. Alrehaili, Reem M. ElKady
Supervision: Ruba A. Alrehaili, Reem M. ElKady
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 July 2024

A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability
- Mirella Vinci 1 na1 ,
- Simone Treccarichi 1 na1 ,
- Rosanna Galati Rando 1 ,
- Antonino Musumeci 1 ,
- Valeria Todaro 2 ,
- Concetta Federico 3 ,
- Salvatore Saccone ORCID: orcid.org/0000-0001-7568-5823 3 ,
- Maurizio Elia 1 &
- Francesco Calì 1
Scientific Reports volume 14 , Article number: 15848 ( 2024 ) Cite this article
Metrics details
- Cellular neuroscience
- Neurological disorders
- Next-generation sequencing
E3 ubiquitin protein ligase encoded by ARIH2 gene catalyses the ubiquitination of target proteins and plays a crucial role in posttranslational modifications across various cellular processes. As prior documented, mutations in genes involved in the ubiquitination process are often associated with autism spectrum disorder (ASD) and/or intellectual disability (ID). In the current study, a de novo heterozygous mutation was identified in the splicing intronic region adjacent to the last exon of the ARIH2 gene using whole exome sequencing (WES). We hypothesize that this mutation, found in an ASD/ID patient, disrupts the protein Ariadne domain which is involved in the autoinhibition of ARIH2 enzyme. Predictive analyses elucidated the implications of the novel mutation in the splicing process and confirmed its autosomal dominant inheritance model. Nevertheless, we cannot exclude the possibility that other genetic factors, undetectable by WES, such as mutations in non-coding regions and polygenic risk in inter-allelic complementation, may contribute to the patient's phenotype. This work aims to suggest potential relationship between the detected mutation in ARIH2 gene and both ASD and ID, even though functional studies combined with new sequencing approaches will be necessary to validate this hypothesis.
Introduction
Genomic variants located outside of the canonical splicing sites (± 2) can lead to abnormal mRNA splicing and are classified as non-canonical splicing variants (NCSVs). The clinical implications of NCSVs in neurodevelopmental disorders (NDDs) remain largely unexplored. Recent studies have underscored the role of intronic variants in disrupting critical neurodevelopmental pathways, suggesting their potential involvement in conditions such as autism spectrum disorder (ASD) and intellectual disability (ID) 1 , 2 , 3 . Among the potentially affected pathways, those regulated by ubiquitination enzymatic activity may be linked to the onset of neurodevelopmental disorders 4 . However, it is important to note that the genetic mechanisms linking non-coding DNA regions to ubiquitination and their impact on neurodevelopmental disorders remain unknown. Detecting smaller, often multiple copy number variants (CNVs) affecting putative regulatory elements may provide insights into additional risks associated with simplex neurodevelopmental disorders, including autism 5 , 6 .
Protein ubiquitination is a posttranslational modification encompassing various fundamental cellular processes 7 . Currently, about 600–700 ubiquitin ligase genes have been detected, representing ~ 5% of the human genes. Protein ubiquitination involves a sophisticated enzymatic cascade comprising ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin ligase (E3) enzymes UBE 2O in the regulation of protein ubiquitination, cellular functions, and disease onset. FEBS J. 286, 2018–2034 (2019)." href="/articles/s41598-024-66475-2#ref-CR8" id="ref-link-section-d96791031e528">8 . E1 enzymes initiate the ubiquitination process by activating ubiquitin through ATP-dependent adenylation. The activated ubiquitin is subsequently transferred to a E2 enzyme, which acts as an intermediary delivering the ubiquitin to a E3 ligase 9 . This highly coordinated process ensures the precise attachment of ubiquitin molecules to target proteins, orchestrating a diverse array of cellular responses and functions 10 , 11 . E2-E3 complexes play a pivotal role in this process, orchestrating the attachment of ubiquitin chains to proteins. The fate of ubiquitinated proteins is determined by the type of ubiquitin chain formed. This intricate regulatory system highlights the multifaceted impact of protein ubiquitination on cellular function.
The ubiquitin–proteasome system (UPS) regulates cellular protein levels crucial for neuronal growth and function, impacting key processes in the nervous system 12 . Mutations in E3 ligase genes are associated with various neurological conditions 13 . In fact, ubiquitination is intricately linked to autism spectrum disorder (ASD), neurodevelopmental impairments, and intellectual disability (ID) 9 . Understanding the UPS's role in these disorders provides insights into their molecular mechanisms, influencing neural development and signalling pathways like WNT, mTOR, and TGFβ. UPS activity directly influences axonal and dendritic development, synaptic maturation, and pruning 14 , 15 , 16 . E3 ligase genes are implicated in ASD, schizophrenia, ID, epilepsy, Parkinson’s disease, and Angelman syndrome, highlighting their broad impact on neurological health 9 , 13 , 17 , 18 , 19 , 20 .
E3 enzymes are categorized into three distinct groups: the really interesting new gene (RING) group, the homologous to E6-AP carboxyl terminus (HECT), and the RING-between-RING (RBR) E3 family. RING ligases operate by directly transferring ubiquitin from E2 enzymes to the substrate through substrate hydrolysis. In contrast, HECT and RBR E3 ligases follow a distinct mechanism. They catalyse ubiquitin transfer to a lysine residue on the E3 ligase itself before subsequently transferring it to the target substrate 9 . Within this context, Ariadne RBR E3 ubiquitin protein ligase 2 (ARIH2) is a RBR family member, which exhibits unique E3 ligase activity, interacting with Ubiquitin-conjugating enzyme E2 L3 (UBE2L3) and functioning similarly to HECT-type E3 enzymes. Particularly, ARIH2 catalyzes ubiquitination of target proteins together with UBE2L3 21 , 22 . These enzymes bind E2s through the first RING-type zinc finger, requiring an obligate trans-thiolation step for ubiquitin transfer involving a conserved cysteine residue in the second RING-type zinc finger 23 .
The E3 ubiquitin protein ligase enzyme encoded by ARIH2 gene comprises 8 highly conserved domains, encompassing three zinc finger type domains: RING-type 1, IBR-type and RING-type 2 24 . As previously documented, E3 ubiquitin protein ligase is autoinhibited by the Ariadne domain, which masks the active site within the second RING-type zinc finger domain, thereby inhibiting E3 activity 19 . Within this context, ARIH2 ubiquitinates substrates of E3 ubiquitin ligase complexes composed of the neddylated forms of Cullin 5 and RING-box protein 2 (neddylated CUL5-RBX2 E3s). Structural analyses reveal ARIH2's distinctive autoinhibition and activation upon association with neddylated CUL5-RBX2, interacting with CUL5 and NEDD8 25 , 26 .
ARIH2 gene is intricately involved in embryonic development through its direct interaction with Hedgehog signalling and myelopoiesis process 27 , 28 . In particular, it directly interplays with Hedgehog signalling through smoothened ubiquitylation and endoplasmic reticulum—associated degradation 28 . According to documented evidence, ARIH2 gene is ubiquitously expressed, showing its highest expression levels in granulocytes 29 . Additionally, ARIH2 shows high expression patterns in several brain tissues, including amygdala, basal ganglion, cerebellar hemispheres, cerebellar vermis, and cerebral cortex (The Human Protein Atlas). Disorders in these brain regions were previously described as potentially involved to neurodevelopmental diseases including ASD and ID 30 , 31 , 32 . ARIH2 is a paralog of ARIH1 gene, and both participate in DNA repair against genotoxic stress and potentially mediate mRNA translation arrest due to its shared Ariadne domain 33 . The human homolog of Ariadne and counterparts in Drosophila and Caenorhabditis elegans are implicated in regulating translation, cellular proliferation, and developmental processes 34 , 35 .
The objective of this study was to verify a new potential relationship between ASD and ID and a de novo novel mutation in an intronic splicing region adjacent to the last exon of ARIH2 gene. This report contributes to further reinforce the knowledge of the genetic basis related to ASD and ID, suggesting the possible involvement of the Ubiquitin E3 ligase pathway in the manifestation of these neurodevelopmental disorders. Additionally, it raises the possibility that mutations in intronic regions, which are often undetectable by WES, may significantly contribute to the patient's phenotype.
Clinical report
The 20-year-old girl was the second daughter of healthy, non-consanguineous parents. She was born at term; no complications occurred during pregnancy. The birth weight registered was 3100 g (z-score: − 0.3), the length was 52 cm (z-score: 1.5) At birth, she presented jaundice, that did not require phototherapy. She presented psychomotor delay: autonomous walking occurred at 20 months of age, first words at 18–20 months of age, sphincter control at the age of 5. When she was 2 years old, the first relational abnormalities were noted: she tended to hide behind her mother, when called, she didn’t promptly answer. At the kindergarten, at the age of 3, she was very shy, spoke softly, she used to play putting the dolls one behind the other, sometimes she launched them. Subsequently, she started to manifest behavioral problems: anxiety reactions, mood instability, repetitiveness, a strong addiction for the mum, episodes of loss of control, hand stereotypies, nocturnal hyperphagia. She was diagnosed of ASD at the age of 10 and treated with quetiapine, risperidone, lamotrigine, cariprazine, without benefit. Brain MRI was unremarkable.
In the 7 months preceding the admission to our department, at the age of 19, she presented frequent episodes (more times per week), of feeling of anxiety, cough, vomiting, headache, lasting for many hours. A slowing in movements and a delay before starting to speak were also noted at that time.
At the age of 20, the clinical phenotype was characterized by downwards-pointing eyelid fissures, posterior auricles, kyphoscoliosis, hand-stereotypies, accentuated during speech, bilateral postural tremor and tendency to keep the hands flexed during walking. Her weight was 54 kg (25–50 centile), her height was 151.5 cm (3–10 centile), her BMI was 23.68 kg/m 2 , head circumference was 55 cm (50–75 centile). At that time, she was treated with haloperidol 2 mg/die, melatonin 2 mg/die. In order to manage her behavioral symptoms, olanzapine was added to her therapy, at the dosage of 10 mg/die, with improvement. The EEG showed sharp-waves and spikes over the temporal regions of the left hemisphere, with tendency to contralateral propagation. Furthermore, magnetic resonance imaging (MRI) of the brain displayed normal results. From a psychological point of view, the diagnosis of ASD level 2 was confirmed, and a diagnosis of moderate ID was made. Comparative genome hybridization (CGH) array didn’t reveal any CNV alterations.
Genetic analysis by next generation sequencing
Whole exome sequencing revealed the presence of a heterozygous de novo nucleotide variation at position c.1411-3C > T, within the last intron in the ARIH2 gene (NM_006321) (Fig. 1 ).

( a ) Graphical representation of the heterozygous de novo variation identified within ARIH2 gene. Images display data from the patient (Pt), the mother (Mo), and the father (Fa). The novel mutation, detected using the Integrative Genome Viewer (IGV) system, is illustrated. At the bottom is displayed the nucleotide sequence and the corresponding amino acids of the region. ( b ) The de novo mutation was confirmed through conventional Sanger sequencing in the patient (Pt), the mother (Mo), and the father (Fa).
Sanger sequencing on the parents confirmed the presence of this mutation only in the patient affected by ASD (Fig. 1 ). WES analysis unveiled no additional mutations directly or potentially associated with other known ASD and/or ID related genes. Furthermore, to confirm that the observed mutation can be considered a de novo mutation, short tandem repeat (STR) polymorphisms were analysed in the patient/mother/father subjects, confirming their parenthood.
The mutation is situated at -3 base pairs from the terminal exon, which encodes the final segment of the Ariadne domain (amino acids 359 to 493) as depicted in Fig. 2 . Conversely, the nucleotide variation observed resulted in a change, from T to C, within the splicing region of donor site sequence, adjacent to the last exon. The causative pathogenetic effect of this specific variant was obtained on the assessment conducted using the ACMG criteria and, more specifically, three criteria for the classification were used, supporting (PP4), moderate (PM2), and strong (PS2) association.

( a ) Depiction of the ubiquitination mechanism. Specifically, this process involves E1, E2, and E3 enzymes. Precisely, E1 initiates the process by the ubiquitin activation (step 1). Subsequently, the activated ubiquitin is transferred to an E2 enzyme (step 2), which delivers it to an E3 ligase (step 3). This process ensures the targeted attachment of ubiquitin to a protein substrate, regulating various cellular functions. ( b ) Autoinhibition of the E3 ubiquitin protein ligase activity operated by the Ariadne domain. Specifically, this domain blocks the second RING-type zinc finger domain, thereby inhibiting E3 activity. Cullin-5 (CUL5) and NEDD8 (N8) are core components of the E3 ubiquitin-protein ligase complex which mediate the proteasomal degradation of target proteins 26 . The graphical representation is a modified version of a previous model related to ARIH1 (whose gene is paralog of ARIH2) having the same domains pattern organization: acidic residues (AC)-UBA-RING1-IBR-RING2-Ariadne 36 . ( c ) Graphical representation of the 8 highly conserved domains within the ARIH2 protein. The figure also highlights the specific position of the identified mutation (c.1411-3C > T), that likely affects the encoding region of the ARIH2 protein from amino acid 471, within the last portion of the Ariadne domain. The figure was modified from the Uniprot protein database.
In silico splicing prediction
In-silico splicing prediction analysis was performed considering the putative new donor/acceptor splicing sites identified close to the start of the exon 16 of the ARIH2 gene (Fig. 3 ). The analysis taken in consideration the new putative donor site “gt”, directly obtained by the variant c.1411-3C > T, further to the putative acceptor site “ag” at the position 48,983,157 (Fig. 3 b,c). The putative "gt" donor site formation, in addition to the predicted constitutive acceptor "ag" (located 38 base pairs upstream of the mutation site), may introduce a new exon codifying for 11 amino acids, due to the following “TGA” Stop codon (Fig. 3 c).

In silico splicing predictions related to the variant c.1411-3C > T in the ARIH2 gene. ( a ) Genomic sequence of the exon 15 and 16, and the intron between them, of the ARIH2 gene. Exon and intron sequences are in uppercase and lowercase letters, respectively. The red lines indicate the translated sequences. The “ag” blue light and the “gt” green nucleotides indicate the acceptor/donor splicing sites respectively. The TAA sequence in a red box indicate the stop codon. On the left, the genomic position, on the chromosome 3, of the first nucleotide on the same line, were indicated. The red “c” nucleotide indicated the site involved in the c.1411-3C > T variant. ( b ) Genomic sequence of the exon 16 and the last part of the intron with the c.1411-3C > T variant (red nucleotide) showing the “ag” (red circle) and the “gt” (blue circle) putative splicing sites. The blue light box indicates the acceptor splicing site determining the wild type mRNA formation. ( c ) Genomic sequence as in ( b ). The blue light box indicates the acceptor splicing site determining, if used, a different mRNA formation, which include a sequence with a stop codon (indicated by the red box) after 11 codons. Red circle indicates the “ag” splicing sites here not used. ( d ) Schematic representation of the two mRNA variants, and the relative amino acid codified, on the basis to the use of the two “ag” acceptor sites shown in “b”, and ( c ). Alt. Exon 16: alternative exon 16 present in the mRNA in the case the “ag” splicing site at position 48,983,157 is used. Exons, introns, and mRNA are not to scale.
The predictive analysis performed with DOMINO described this gene as having an autosomal dominant (AD) inheritance pattern (probability of AD: 0.6318). Furthermore, the variant was classified by FATHMM, Reg-SNP-intron and Mutation taster algorithms as disease causing (Table 1 ).
In-silico splicing prediction analysis conducted using ASSP and Spliceator algorithms revealed that the identified variant created a novel “gt” donor gain site, as result of the genetic variant c.1411-3C > T within ARIH2 (Fig. 3 ), further to the constitutive acceptor sites "ag", located 38 base pairs upstream of the mutation site. Both utilized tools categorized the variant site as a potential new alternative isoform or cryptic donor (Fig. 3 and Table 2 ). The newly generated donor site as a consequence of the mutation exhibits scores of 4.554 and 0.711 for ASSP and Spliceator, respectively. In addition, the prediction indicated the presence of a plausible constitutive acceptor site "ag" located 38 base pairs upstream of the mutation site exhibiting high scores (9.280 and higher than 0.7 according to ASSP and Spliceator, respectively) (Table 2 ). As predicted, both acceptor and donor sites may introduce a new exon consisting of 11 amino acids and a “TGA” Stop codon (Fig. 3 ).
In addition, the analyses conducted using NNSplice tool and Mutation Taster, revealed a candidate donor site alteration in the intronic region adjacent to the last exon of the ARIH2 gene resulting with a splice site gain score of 0.37 (Table 3 ). Furthermore, PhyloP score exhibited the value of 1.58, indicating a moderate grade of conservation of the analysed splicing region. Conversely, PhastCons score for the splicing region at the mutation site was 0.914 (Table 3 ).
Hydrogen bonds assessment
The analysis conducted using UCSF ChimeraX revealed a total of 42 hydrogen bonds within the region of the Ariadne domain, specifically corresponding to the segment affected by the mutation spanning from amino acid 471 to 493. These electrostatic bonds spanned between intra and extra Ariadne domain (359–493 aa). Notably, six of these interactions involved domains other than the Ariadne one (Fig. 4 ). Specifically, these bonds included interactions between the residues His491 (one hydrogen bond) and Asp492 (two hydrogen bonds) with Tyr264 and Arg245, respectively. Remarkably, both residues were situated within the zinc finger RING 1 domain. Additionally, these electrostatic interactions implicated the residues Arg483 (two hydrogen bonds) and Arg484 (one hydrogen bond), with interactions occurring between Glu57 (comprising an extra domain region) and Glu47 (UBA-like domain), respectively. STRING analysis revealed the correlation between E3 ubiquitin protein ligase and NEDD8, CUL5, RFN7 and UBE2L3 proteins. Specifically, these correlations were based on experimental evidence.

( a ) Wild type and ( b ) mutated E3 ubiquitin protein ligase of ARIH2 gene. ( c - f ) Ariadne domain: graphical representation of amino acid residues involved in hydrogen bond interactions in the last portion of the Ariadne domain (from 471 to 493 aa), including the last helix of the Ariadne domain (comprising from aa 469 to aa 491). This gene was affected by a de novo mutation in the intronic region at − 3 bp from the exon 16 of ARIH2 gene. The figure illustrates six hydrogen bonds involving the region spanning from 471 to 493 aa and extra Ariadne domain residues: ( c ) one hydrogen bond between residues HIS491 and TYR264 (zinc finger RING 1 domain); ( d ) two hydrogen bonds between residues ASP492 and ARG245 (zinc finger RING 1 domain); ( e ) one hydrogen bond between residues ARG484 and GLU47 (UBA-like domain); ( f ) two hydrogen bonds between residues ARG483 and GLU57. The images were realized using the software UCSF Chimera X.
Genetic variants outside the canonical splicing sites can disrupt normal mRNA splicing, potentially impacting neurodevelopmental disorders clinically. Furthermore, mutations in genes involved in the ubiquitination process are often associated with ASD and/or ID 37 , 38 . The UPS intricately interplays with the non-lysosomal proteolytic pathway regulating cellular protein levels. This regulatory mechanism extends to proteins crucial for neuronal growth and function, emphasizing UPS's central role in modulating key cellular processes within the nervous system 12 .
Here we report details on the identification of a de novo, heterozygous mutation (c.1411-3C > T) localized in the splicing region (at − 3 bp from the last exon) in ARIH2 gene, detected through next generation sequencing. This variation was detected in a patient exhibiting both ASD and ID. To date, ARIH2 doesn’t have an assigned OMIM number and code. Nevertheless, the predictive analysis performed with DOMINO identified ARIH2 gene as having an autosomal dominant (AD) inheritance pattern (probability of AD: 0.6318). The comprehensive whole exome analysis revealed no additional mutations directly or potentially associated with other known ASD and ID related genes. However, we cannot exclude the possibility that other genetic factors, undetectable by WES, may contribute to the phenotype. This specific variant has never been described in gnomAD and 1000G databases. We suggest a potential relationship between the pathogenic effect of this specific variant, based on the assessment conducted using the ACMG criteria. Additionally, the in silico analysis conducted using the Mutation Taster, FATHMM, and RegSNPs-intron tools revealed a potential pathogenic significance of the identified variant (Table 1 ).
Moreover, in silico splicing predictions conducted using the NNSplice, ASSP, and Spliceator algorithms revealed with high score that the identified variant (c.1411-3C > T) created a novel donor gain site, represented by the mutated "GT" sequence. In addition, the prediction analysis performed, indicated the presence of a plausible constitutive acceptor site "AG" located 38 base pairs upstream of the mutation site exhibiting high scores (Fig. 3 , and Table 2 ). As predicted both acceptor and donor sites may introduce a new exon. This exon consists of 11 amino acids and a “TGA” stop codon.
For further support these findings, Mutation Taster yielded positive scores for both PhyloP and PhastCons, indicating a moderate and high conservation rate, respectively, at the specific site of the variant. Indeed, "GT" is a well-established sequence often associated with alternative donor sites in eukaryotic splicing 39 . Based on this prediction, we hypothesize that the splicing process alteration may disrupt the encoding of the last protein portion, resulting in a misfolded E3 ubiquitin protein ligase. Furthermore, it's important to note that intronic variants at position -3 in genes like NF1 , TSC1 , and PAH , (as indicated by HGMD database) constitute approximately 4% of causative mutations within intronic regions. This underscores the significance of exploring such variants in elucidating their potential contributions to pathogenicity, despite the limitations related to the possible presence of other variants not identified by WES analysis. We emphasize that a comprehensive understanding of the alternative splicing process would necessitate experimental studies on mRNA/cDNA, which, regrettably, are not currently possible.
Our hypothesis emphasizes that this splicing mutation alters the polypeptide chain of E3 ubiquitin protein ligase enzyme altering the last portion of the Ariadne domain (Fig. 2 and Fig. 4 ). The prediction of hydrogen bonds in the disrupted portion of the ARIH2 protein revealed the engagement of six significant hydrogen bonds between this region (471–493 aa) and other functional domains than Ariadne. Based on these findings, we propose that the absence of these electrostatic interactions has an impact on protein function. We underscore that the protein structure prediction conducted with UCSF ChimeraX considers elements such as the arrangement of alpha helices, beta strands, and loops. However, as limiting factor, it may not provide precise explicit modeling of metal ion binding sites like Zn 2+ . Nonetheless, as the prediction leverages the AlphaFold deep learning algorithm, it might indirectly capture certain metal ion interactions.
As previously documented, E3 ubiquitin protein ligase is autoinhibited by the Ariadne domain, which masks the active site within the second RING-type zinc finger domain, thereby inhibiting E3 activity 36 . Ariadne domain in Drosophila and Caenorhabditis elegans within ARIH1 gene (paralog of ARIH2 ) have been implicated in the transcriptional and translational regulation 34 , 35 . As previously reported 40 , these processes, in a diverse group of genes, were associated with both syndromic and non‐syndromic forms of ASD. Despite ARIH1 and ARIH2 deriving from a common ancestral gene, their protein sequences exhibit low homology (34.91% overall and 38.06% in the Ariadne domain). Nevertheless, they have the same domains pattern organization (AC-UBA-RING1-IBR-RING2-Ariadne). While limited structural information exists on the IBR domain, protein structure predictions suggest potential hydrogen bond formation between the IBR and Ariadne domains (specifically between Asp 492 and Arg 245, as well for His 491 and Tyr 264) (Fig. 4 ). Given the structural proximity identified between the Ariadne and IBR domains, these electrostatic interactions seem plausible based on our protein structure prediction analysis 41 , 42 , 43 . Building upon these hypotheses, it is likely that the disruption of the Ariadne domain significantly impacts, altering post-transcriptional modifications of the target protein, which might potentially contribute to the development of ASD and ID.
As previously documented, E3 ligases genes have been linked to neurological disorders that include neurodevelopmental or neurodegenerative diseases, such as autism, schizophrenia, intellectual disability, epilepsy, Angelman syndrome, and Parkinson’s disease 9 , 13 , 17 , 18 , 19 , 20 . As was outlined, the alteration of the E3 ubiquitin protein ligase structure in patients showing ASD, negatively interacts with synaptic function and plasticity, altering learning and memory formation by targeting activity regulated cytoskeleton-associated protein (Arc), a Rho guanine nucleotide exchange factor (Ephexin5), and small conductance calcium-activated potassium channel (SK2) 37 , 44 , 45 . In particular, several E3 ligases are encoded by various genes, such as UBE3A , UBE3B , TRIP12 and HUWE1 showing specific pathogenic variations associated to Autism 37 , 38 , 46 .
Interestingly, ARIH2 gene expression was observed in several brain tissues, including amygdala, basal ganglion, cerebellar hemispheres, cerebellar vermis and cerebral cortex, tissues potentially involved in ASD 30 , 31 , 32 , 47 . Moreover, previous studies indicated a differential expression of ARIH2 in post-mortem cerebral cortex tissue between individuals with ASD and neurotypical controls. Specifically, ARIH2 was found to be downregulated in ASD patients 48 , 49 .
ARIH2 was investigated in a cohort of patients analyzed for the detection of rare coding variations by population genetic approach, aiming to provide valuable insights into the genetic architecture and phenotypic context of autism 50 . Within this context, two de novo variants were detected in 5’ UTR and intronic regions of ARIH2 gene, exhibiting an uncertain correlation with autism. It’s worth mentioning that an additional de novo mutation, in the ARIH2 gene, involving a repeated motif AAG-1 was previously reported in a proband affected by ASD 51 . A prior study conducted a predictive analysis focused on loss-of-function mutations in six genes, considering factors such as the extent of brain expression, haploinsufficiency index, functional knowledge about gene homology, and insights from mouse models. This analysis identified ARIH2 as a candidate gene associated with ID 52 . Furthermore, ARIH2 was postulated to be part of a subset of intelligence-related genes, characterized by intra-species variations in human populations. These genes may also be evolution-related, exhibiting inter-species variations between humans ( Homo sapiens ) and great apes (including P. troglodytes and P. abelii ) 53 .
The analysis performed with STRING based on experimental evidence of the protein pathways through the study of protein–protein interactions, revealed a significant allosteric specificity among ARIH2, CUL5 and NEDD8 genes. The latter was reported as likely associated to ASD 45 . Specifically, all these genes ( ARIH2 , NEDD8 , CUL5 ) interplay with the conjugation and ubiquitination activities mediating the subsequent proteasomal degradation of target proteins (Fig. 2 b). Notably, NEDD8 uniquely contacts covalently linked CUL5 , inducing structural rearrangements that expose previously hidden regions of ARIH2 26 . Furthermore, there is evidence supporting the role of NEDD8 and CUL5 in regulating mammalian excitatory synapses and neural development 54 , 55 .
Notably, we are hypothesizing that the splicing mutation that we detected might be associated with ASD and ID similarly to the prior studied mutations in genes encoding for E3 ubiquitin protein ligase. Additionally, it is important to emphasize a potential limitation: variants in non-coding regions, such as those associated with promoter regions, may also contribute to the ASD phenotype 56 . Within this context, it is essential to acknowledge that while Whole Exome Sequencing (WES) is a powerful tool for identifying coding mutations, it may not capture genetic variations in non-coding regions or complex polygenic interactions 1 , 57 . These undetected mutations, including those in non-coding regions and polygenic risks arising from inter-allelic complementation, could potentially play significant roles in shaping the patient's phenotype 58 . A limitation of our WES analysis is the filtering out of polymorphisms with Minor Allele Frequencies (MAF) higher than 1%, which may contribute to polygenic risk. The limitations of WES in detecting these variants underscore the need for complementary approaches, such as Whole Genome Sequencing (WGS) or targeted sequencing of specific regulatory elements, to fully elucidate the genetic landscape underlying complex neurodevelopmental disorders 59 , 60 . Integrating these techniques with advanced bioinformatic analyses will be crucial for uncovering the complete spectrum of genetic factors contributing to the phenotype.
Although several studies have identified the strong role of ubiquitination in autism and intellectual disability, the association between ARIH2 gene and these conditions that we are proposing is only a hypothesis. In fact, further patients exhibiting ASD or ID with variants in the ARIH2 gene are needed to validate our study. Further in vitro functional assays on ARIH2 gene are essential to test the validity of the novel gene and to enhance our understanding of the complex interactions among genes encoding proteins in ASD.
Understanding ubiquitination's role in cellular processes offers insight into disease mechanisms and therapeutic avenues. Drugs targeting ubiquitin enzymes can modulate protein degradation, suggesting potential therapeutic interventions 61 , 62 , 63 . Therefore, comprehending ubiquitination and its implications in cellular physiology and pathology can lay the basis for the development of novel therapeutic approaches for various diseases.
In this study, we report a patient showing both ASD and ID. Whole exome sequencing (WES) enabled the identification of a de novo heterozygous mutation in the ARIH2 gene encoding for an E3 ubiquitin protein ligase. Notably, this novel mutation is located in the intronic region adjacent to the last exon of this gene. Based on a comprehensive assessment of its plausible pathogenic role, we hypothesize a potential relationship between ARIH2 and the patient's phenotype. Nevertheless, we cannot rule out the possibility that other genetic factors, which are undetectable by WES, could determinate or contribute to the patient's phenotype. It is worth mentioning that E3 ubiquitin ligases are a large family of enzymes widely documented as implicated in ASD and ID. Our hypothesis is that the mutation alters the last portion of the Ariadne domain involved in autoinhibition of the E3 ubiquitin ligase protein modulating proteasomal degradation. ARIH2 gene lacks an assigned OMIM number. We propose for the first time an association between this gene and ASD/ID. Further functional studies are needed in autistic patients exhibiting ARIH2 variants, to validate the current study.
Materials and methods
Library preparation and ngs analysis.
Genomic DNA was isolated from peripheral blood leukocytes obtained from the clinical case, as well from the father and the mother. The extraction protocol applied was a non-organic and non-enzymatic extraction method previously developed 64 . Exome analysis was performed using the Ion AmpliSeq™ Exome RDY kits, following the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). The quality of libraries was assessed using DNA 1000 chips on the Tape Station 4200 (Agilent, Santa Clara, CA, USA) and Qubit dsDNA BR Assay kits (Invitrogen, Waltham, MA, USA). Template preparation, clonal amplification, recovery, and enrichment of template-positive Ion Sphere™ particles and loading of sequencing-ready Ion Torrent semiconductor chips were performed with the Ion Chef™ system (Thermo Fisher Scientific, Waltham, MA, USA). Finally, we sequenced each loaded Ion 550™ chip on the S5 system (Thermo Fisher Scientific, Waltham, MA, USA). Overall, 98% of regions of interest have a minimum coverage of at least 20 × . Data of runs were processed using the Ion Torrent Suite 5.16, Variant Caller 5.16, Coverage Analysis 5.16 (Thermo Fisher Scientific, Waltham, MA, USA), Ion Reporter (Thermo Fisher Scientific, Waltham, MA, USA), and/or wANNOVAR tools 65 . DNA sequences were displayed using Integrated Genomics Viewer 66 . The reference genome employed for the analysis was Hg38. Pathogenic variant was confirmed through conventional Sanger sequencing. The methods related to the Variant Caller parameters and PCR setup were available in the Supplementary File 1. DNA fingerprint analysis was performed for both patient and parents, according to a previous protocol 67 , to confirm maternity and paternity, and de novo event.
Data analysis
The observed nucleotide variation was classified according to the “American College of Medical Genetics” (ACMG) guidelines 68 and it was performed with VarSome 69 , and the three criteria used for the classification used were reported in Table 4 .
For the in silico evaluation of the pathogenic significance of the variant, we utilized the MutationTaster algorithm ( https://www.mutationtaster.org/ ) (accessed on 15 November 2023) along with the FATHMM ( https://fathmm.biocompute.org.uk/ ) and RegSNPs-intron ( https://regsnps-intron.ccbb.iupui.edu/ ) tools (accessed on 17 April 2024). These tools assign output values ranging from 0 to 1, indicating the pathogenic significance of the variant 70 , 71 .
Protein sequence and domains were obtained from the free online database of UniProt ( https://www.uniprot.org/ ) (accessed on 15 November 2023) (UniProt Consortium, 2015). Expression level was ascertained on The Human Protein Atlas ( https://www.proteinatlas.org/ ) (accessed on 15 November 2023) 72 .
The in silico prediction of the splicing process was performed using NNSplice tool, provided by Mutation Taster website, in addition to ASSP ( http://wangcomputing.com/assp/ ) and Spliceator ( https://www.lbgi.fr/spliceator/ ) tools (accessed on 17 April 2024). ASSP scores range from 0 to 10, with higher scores indicating increased likelihood of a splice site. Cutoff values of 2.2 for acceptor sites and 4.5 for donor sites achieve correct identification rates of approximately 75% and 80%, respectively, for false and alternative isoform/cryptic splice sites 73 , 74 . Spliceator scores range from 0 to 1, with higher scores indicating higher likelihood of a sequence being recognized as a splice site 75 .
The confidence, PhyloP and PhastCons scores were analysed according to previous studies 76 , 77 , 78 . Notably, a mutation taster splice site gain score higher than 0.3 indicates gain of a completely new splice site. Conversely, PhyloP and PhastCons scores assess conservation rate in the analysed region. Within this context, PhyloP score spans between -14 and 6. Sites predicted to be conserved are assigned positive scores, while sites predicted to be fast evolving are assigned negative scores. On the other hand, PhastCons range between 0 and 1.
Additionally, in silico conservation rate was analyzed by DOMINO with the score provided by VarSome database (accessed on 15 November 2023) 69 , according to prior research 79 , 80 . For all the bioinformatic tools employed were used the default parameters. The Human Gene Mutation Database (HGMD) ( https://www.hgmd.cf.ac.uk/ ) (accessed on 17 April 2024) 81 was utilized to determine the percentage of intronic variants located at position -3 from various exons, which have been confirmed to have causative effects in genes such as NF1, TSC1, and PAH. STRING analysis was conducted for analyzing the protein pathways correlated with ARIH2 gene based on experimental studies ( https://string-db.org/ ) (accessed on 15 November 2023) 82 .
Molecular graphics and analyses performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases 83 .
Institutional review board statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee “Comitato Etico IRCCS Sicilia-Oasi Maria SS”. Prot. CE/88bis, as of 20 March 2019, approval code: 2019/03/18/CE-IRCCS-OASI/18.
Informed consent statement
Written informed consent has been obtained from the patient’s parents to publish this article.
Data availability
The datasets analysed during the current study are available in the ClinVar repository, acc. No. VCV002692232.1 (Variation ID: 2692232). [Web link: https://www.ncbi.nlm.nih.gov/clinvar/variation/2692232/?oq=2692232&m=NM_006321.4(ARIH2):c.1411-3C%3ET ]. Figure 2 C data are from Uniprot protein database. Accession: https://www.uniprot.org/uniprotkb/O95376/entry , family & domains section. Accessed on 19/02/2024.
An, J.-Y. et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science https://doi.org/10.1126/science.aat6576 (2018).
Article PubMed PubMed Central Google Scholar
Dhaene, E. & Vergult, S. Interpreting the impact of noncoding structural variation in neurodevelopmental disorders. Genetics in Medicine 23 , 34–46 (2021).
Article CAS PubMed Google Scholar
Li, K. et al. Prioritizing de novo potential non-canonical splicing variants in neurodevelopmental disorders. EBioMedicine 99 , 104928 (2024).
Manoubi, W. et al. Genetic investigation of the ubiquitin-protein ligase E3A gene as putative target in Angelman syndrome. World J. Clin. Cases 12 , 503–516 (2024).
Turner, C. M. et al. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol. Dial. Transplant. 22 , 386–395 (2006).
Article PubMed Google Scholar
Pagnamenta, A. T. et al. Structural and non-coding variants increase the diagnostic yield of clinical whole genome sequencing for rare diseases. Genome Med. 15 , 94 (2023).
Article CAS PubMed PubMed Central Google Scholar
Schmidt, M. F., Gan, Z. Y., Komander, D. & Dewson, G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ. 28 , 570–590 (2021).
Ullah, K., Zubia, E., Narayan, M., Yang, J. & Xu, G. Diverse roles of the E2/E3 hybrid enzyme <scp>UBE</scp> 2O in the regulation of protein ubiquitination, cellular functions, and disease onset. FEBS J. 286 , 2018–2034 (2019).
Kasherman, M. A., Premarathne, S., Burne, T. H. J., Wood, S. A. & Piper, M. The Ubiquitin System: a Regulatory Hub for Intellectual Disability and Autism Spectrum Disorder. Mol. Neurobiol. 57 , 2179–2193 (2020).
Martínez-Noël, G. et al. Network Analysis of UBE3A/E6AP-Associated Proteins Provides Connections to Several Distinct Cellular Processes. J. Mol. Biol. 430 , 1024–1050 (2018).
Toma-Fukai, S. & Shimizu, T. Structural Diversity of Ubiquitin E3 Ligase. Molecules 26 , 6682 (2021).
Crider, A., Pandya, C. D., Peter, D., Ahmed, A. O. & Pillai, A. Ubiquitin-proteasome dependent degradation of GABAAα1 in autism spectrum disorder. Mol. Autism. 5 , 45 (2014).
George, A. J., Hoffiz, Y. C., Charles, A. J., Zhu, Y. & Mabb, A. M. A Comprehensive Atlas of E3 Ubiquitin Ligase Mutations in Neurological Disorders. Front. Genet. https://doi.org/10.3389/fgene.2018.00029 (2018).
Kumar, D., Ambasta, R. K. & Kumar, P. Ubiquitin biology in neurodegenerative disorders: From impairment to therapeutic strategies. Ageing Res. Rev. 61 , 101078 (2020).
Zajicek, A. & Yao, W.-D. Remodeling without destruction: non-proteolytic ubiquitin chains in neural function and brain disorders. Mol. Psychiatry 26 , 247–264 (2021).
Tang, G. et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron 83 , 1131–1143 (2014).
Upadhyay, A. et al. E3 Ubiquitin Ligases Neurobiological Mechanisms: Development to Degeneration. Front Mol. Neurosci. https://doi.org/10.3389/fnmol.2017.00151 (2017).
Ardley, H. C. & Robinson, P. A. The Role of Ubiquitin-Protein Ligases in Neurodegenerative Disease. Neurodegener. Dis. 1 , 71–87 (2004).
Hampe, C., Ardila-Osorio, H., Fournier, M., Brice, A. & Corti, O. Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin–protein ligase with monoubiquitylation capacity. Hum. Mol. Genet. 15 , 2059–2075 (2006).
Conway, J. A., Kinsman, G. & Kramer, E. R. The Role of NEDD4 E3 Ubiquitin-Protein Ligases in Parkinson’s Disease. Genes (Basel) 13 , 513 (2022).
Buetow, L. & Huang, D. T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell. Biol. 17 , 626–642 (2016).
Zhang, Y. & Hou, L. Alternate Roles of Sox Transcription Factors beyond Transcription Initiation. Int. J. Mol. Sci. 22 , 5949 (2021).
Scott, D. C. et al. Two Distinct Types of E3 Ligases Work in Unison to Regulate Substrate Ubiquitylation. Cell 166 , 1198-1214.e24 (2016).
Kawashima, A. et al. ARIH2 Ubiquitinates NLRP3 and Negatively Regulates NLRP3 Inflammasome Activation in Macrophages. J. Immunol. 199 , 3614–3622 (2017).
Lamsoul, I., Uttenweiler-Joseph, S., Moog-Lutz, C. & Lutz, P. G. Cullin 5-RING E3 ubiquitin ligases, new therapeutic targets?. Biochimie 122 , 339–347 (2016).
Kostrhon, S. et al. CUL5-ARIH2 E3–E3 ubiquitin ligase structure reveals cullin-specific NEDD8 activation. Nat. Chem. Biol. 17 , 1075–1083 (2021).
Lin, A. E. et al. ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system. Nat. Immunol. 14 , 27–33 (2013).
Lv, B., Zhang, X.-O. & Pazour, G. J. Arih2 regulates Hedgehog signaling through smoothened ubiquitylation and ER-associated degradation. J. Cell Sci. https://doi.org/10.1242/jcs.260299 (2022).
Marteijn, J. A. F. et al. The E3 ubiquitin-protein ligase Triad1 inhibits clonogenic growth of primary myeloid progenitor cells. Blood 106 , 4114–4123 (2005).
Hampson, D. R. & Blatt, G. J. Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. https://doi.org/10.3389/fnins.2015.00420 (2015).
D’Mello, A. M. & Stoodley, C. J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. https://doi.org/10.3389/fnins.2015.00408 (2015).
Prat, C. S., Stocco, A., Neuhaus, E. & Kleinhans, N. M. Basal ganglia impairments in autism spectrum disorder are related to abnormal signal gating to prefrontal cortex. Neuropsychologia 91 , 268–281 (2016).
von Stechow, L. et al. The E3 Ubiquitin Ligase ARIH1 Protects against Genotoxic Stress by Initiating a 4EHP-Mediated mRNA Translation Arrest. Mol. Cell. Biol. 35 , 1254–1268 (2015).
Article Google Scholar
Aguilera, M., Oliveros, M., Martínez-Padrón, M., Barbas, J. A. & Ferrús, A. Ariadne-1: A Vital Drosophila Gene Is Required in Development and Defines a New Conserved Family of RING-Finger Proteins. Genetics 155 , 1231–1244 (2000).
Elmehdawi, F. et al. Human Homolog of Drosophila Ariadne (HHARI) is a marker of cellular proliferation associated with nuclear bodies. Exp. Cell. Res. 319 , 161–172 (2013).
Duda, D. M. et al. Structure of HHARI, a RING-IBR-RING Ubiquitin Ligase: Autoinhibition of an Ariadne-Family E3 and Insights into Ligation Mechanism. Structure 21 , 1030–1041 (2013).
Greer, P. L. et al. The Angelman Syndrome Protein Ube3A Regulates Synapse Development by Ubiquitinating Arc. Cell 140 , 704–716 (2010).
Vatsa, N. & Jana, N. R. UBE3A and Its Link With Autism. Front. Mol. Neurosci. https://doi.org/10.3389/fnmol.2018.00448 (2018).
Furlong, R. Refining the splice region. Nat. Rev. Genet. 19 , 470–471 (2018).
Longo, F. & Klann, E. Reciprocal control of translation and transcription in autism spectrum disorder. EMBO Rep. https://doi.org/10.15252/embr.202052110 (2021).
Moynihan, T. P. et al. The Ubiquitin-conjugating Enzymes UbcH7 and UbcH8 Interact with RING Finger/IBR Motif-containing Domains of HHARI and H7-AP1. J. Biol. Chem. 274 , 30963–30968 (1999).
Eisenhaber, B., Chumak, N., Eisenhaber, F. & Hauser, M.-T. The ring between ring fingers (RBR) protein family. Genome Biol. 8 , 209 (2007).
Dove, K. K. et al. Structural Studies of HHARI/UbcH7 ∼ Ub Reveal Unique E2 ∼ Ub Conformational Restriction by RBR RING1. Structure 25 , 890-900.e5 (2017).
Margolis, S. S. et al. EphB-mediated degradation of the RhoA GEF ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 143 , 442–455 (2010).
Sun, J. et al. UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell. Rep. 12 , 449–461 (2015).
Giles, A. C. & Grill, B. Roles of the HUWE1 ubiquitin ligase in nervous system development, function and disease. Neural Dev. 15 , 6 (2020).
Nordahl, C. W. Increased Rate of Amygdala Growth in Children Aged 2 to 4 Years With Autism Spectrum Disorders. Arch. Gen. Psychiatry 69 , 53 (2012).
Parikshak, N. N. et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 540 , 423–427 (2016).
Matoba, N. et al. Common genetic risk variants identified in the SPARK cohort support DDHD2 as a candidate risk gene for autism. Transl. Psychiatry 10 , 265 (2020).
Fu, J. M. et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 54 , 1320–1331 (2022).
Mitra, I. et al. Patterns of de novo tandem repeat mutations and their role in autism. Nature 589 , 246–250 (2021).
Tallantyre, E. & Robertson, N. P. Autism and intellectual disability. J. Neurol. 260 , 936–939 (2013).
Li, M., Zhang, W. & Zhou, X. Identification of genes involved in the evolution of human intelligence through combination of inter-species and intra-species genetic variations. PeerJ 8 , e8912 (2020).
Simó, S., Jossin, Y. & Cooper, J. A. Cullin 5 Regulates Cortical Layering by Modulating the Speed and Duration of Dab1-Dependent Neuronal Migration. J. Neurosci. 30 , 5668–5676 (2010).
Scudder, S. L. & Patrick, G. N. Synaptic structure and function are altered by the neddylation inhibitor MLN4924. Mol. Cell. Neurosci. 65 , 52–57 (2015).
Nakamura, T. et al. Topologically associating domains define the impact of de novo promoter variants on autism spectrum disorder risk. Cell. Genom. 4 , 100488 (2024).
Lewis, C. M. & Vassos, E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 12 , 44 (2020).
Vinci, M. et al. Exome sequencing in a child with neurodevelopmental disorder and epilepsy: Variant analysis of the AHNAK2 gene. Mol. Genet. Genomic Med. https://doi.org/10.1002/mgg3.2012 (2022).
Abbasi, A. & Alexandrov, L. B. Significance and limitations of the use of next-generation sequencing technologies for detecting mutational signatures. DNA Repair 107 , 103200 (2021).
Tilemis, F.-N. et al. Germline CNV Detection through Whole-Exome Sequencing (WES) Data Analysis Enhances Resolution of Rare Genetic Diseases. Genes 14 , 1490 (2023).
Chen, C., Qin, H., Tan, J., Hu, Z. & Zeng, L. The Role of Ubiquitin-Proteasome Pathway and Autophagy-Lysosome Pathway in Cerebral Ischemia. Oxid. Med. Cell. Longev. 2020 , 1–12 (2020).
CAS Google Scholar
Zhang, L. et al. Dexmedetomidine Mitigated NLRP3-Mediated Neuroinflammation via the Ubiquitin-Autophagy Pathway to Improve Perioperative Neurocognitive Disorder in Mice. Front. Pharmacol. https://doi.org/10.3389/fphar.2021.646265 (2021).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20 , 1242–1253 (2014).
Lahiri, D. K., Bye, S., Nurnberger, J. I., Hodes, M. E. & Crisp, M. A non-organic and non-enzymatic extraction method gives higher yields of genomic DNA from whole-blood samples than do nine other methods tested. J. Biochem. Biophys. Methods 25 , 193–205 (1992).
Chang, X. & Wang, K. wANNOVAR: annotating genetic variants for personal genomes via the web. J. Med. Genet. 49 , 433–436 (2012).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14 , 178–192 (2013).
Calì, F. et al. DXYS156: a multi-purpose short tandem repeat locus for determination of sex, paternal and maternal geographic origins and DNA fingerprinting. Int. J. Legal Med. 116 , 133–138 (2002).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17 , 405–424 (2015).
Kopanos, C. et al. VarSome: the human genomic variant search engine. Bioinformatics 35 , 1978–1980 (2019).
Shihab, H. A. et al. Predicting the Functional, Molecular, and Phenotypic Consequences of Amino Acid Substitutions using Hidden Markov Models. Hum. Mutat. 34 , 57–65 (2013).
Lin, H. et al. RegSNPs-intron: a computational framework for predicting pathogenic impact of intronic single nucleotide variants. Genome Biol. 20 , 254 (2019).
Pontén, F., Schwenk, J. M., Asplund, A. & Edqvist, P.-H.D. The Human Protein Atlas as a proteomic resource for biomarker discovery. J. Intern. Med. 270 , 428–446 (2011).
Wang, M. & Marín, A. Characterization and prediction of alternative splice sites. Gene 366 , 219–227 (2006).
Tang, R., Prosser, D. O. & Love, D. R. Evaluation of Bioinformatic Programmes for the Analysis of Variants within Splice Site Consensus Regions. Adv. Bioinform. 2016 , 1–10 (2016).
Article CAS Google Scholar
Scalzitti, N. et al. Spliceator: multi-species splice site prediction using convolutional neural networks. BMC Bioinform. 22 , 561 (2021).
Pollard, K. S., Hubisz, M. J., Rosenbloom, K. R. & Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 20 , 110–121 (2010).
Schwarz, J. M., Rödelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7 , 575–576 (2010).
Montenegro, L. R., Lerário, A. M., Nishi, M. Y., Jorge, A. A. L. & Mendonca, B. B. Performance of mutation pathogenicity prediction tools on missense variants associated with 46 XY differences of sex development. Clinics 76 , e2052 (2021).
Frías-López, C. et al. DOMINO: development of informative molecular markers for phylogenetic and genome-wide population genetic studies in non-model organisms. Bioinformatics 32 , 3753–3759 (2016).
Quinodoz, M. et al. DOMINO: Using Machine Learning to Predict Genes Associated with Dominant Disorders. Am. J. Hum. Genet. 101 , 623–629 (2017).
Stenson, P. D. et al. The Human Gene Mutation Database (HGMD®): Optimizing its use in a clinical diagnostic or research setting. Hum. Genet. 139 , 1197–1207 (2020).
Crosara, K. T. B., Moffa, E. B., Xiao, Y. & Siqueira, W. L. Merging in-silico and in vitro salivary protein complex partners using the STRING database: A tutorial. J. Proteomics 171 , 87–94 (2018).
Meng, E. C. et al. UCSF ChimeraX : Tools for structure building and analysis. Protein Sci. https://doi.org/10.1002/pro.4792 (2023).
Download references
Acknowledgements
Special acknowledgements for this paper are due to Eleonora Di Fatta for her valuable assistance in the translation, preparation and formatting of the text. . We would like to thank Angelo Gloria, Alda Ragalmuto, and Valeria Chiavetta for their technical contribution.
This work was partially supported by the Italian Ministry of Health “Ricerca Corrente 2017–2023” and 5xmille.
Author information
These authors contributed equally: Mirella Vinci and Simone Treccarichi.
Authors and Affiliations
Oasi Research Institute-IRCCS, 94018, Troina, Italy
Mirella Vinci, Simone Treccarichi, Rosanna Galati Rando, Antonino Musumeci, Maurizio Elia & Francesco Calì
Department of Medical and Surgical Sciences and Advanced Technologies “G.F. Ingrassia”, University of Catania, Catania, Italy
Valeria Todaro
Department of Biological, Geological and Environmental Sciences, University of Catania, Via Androne 81, 95124, Catania, Italy
Concetta Federico & Salvatore Saccone
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: M.V, S.T, F.C.; methodology: M.V, R.G.R, V.T., M.E.; validation: M.V., S.T., R.G.R., F.C.; investigation: M.V., S.T., R.G.R., A.M., V.T., C.F., S.S., M.E., F.C.; data curation: M.V.., S.T., M.E., F.C.; original draft preparation: M.V., S.T., A.M., S.S., M.E., F.C.; manuscript review and editing: M.V., S.T., S.S., M.E., F.C.; supervision: M.V., S.T., S.S., F.C All authors have read and agreed to the final version of the manuscript.
Corresponding author
Correspondence to Salvatore Saccone .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Vinci, M., Treccarichi, S., Galati Rando, R. et al. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci Rep 14 , 15848 (2024). https://doi.org/10.1038/s41598-024-66475-2
Download citation
Received : 20 February 2024
Accepted : 01 July 2024
Published : 09 July 2024
DOI : https://doi.org/10.1038/s41598-024-66475-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ubiquitination
- Whole exome sequencing
- Autism spectrum disorder
- E3 ubiquitin-protein ligase
- Splicing region
- Autosomal dominant inheritance model
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 05 July 2024
Exposure to heavy metals in utero and autism spectrum disorder at age 3: a meta-analysis of two longitudinal cohorts of siblings of children with autism
- John F. Dou 1 ,
- Rebecca J. Schmidt 2 ,
- Heather E. Volk 3 ,
- Manon M. Nitta 1 ,
- Jason I. Feinberg 3 ,
- Craig J. Newschaffer 4 ,
- Lisa A. Croen 5 ,
- Irva Hertz-Picciotto 2 ,
- M. Daniele Fallin 6 &
- Kelly M. Bakulski 1
Environmental Health volume 23 , Article number: 62 ( 2024 ) Cite this article
104 Accesses
1 Altmetric
Metrics details
Autism spectrum disorder (ASD) is a prevalent and heterogeneous neurodevelopmental disorder. Risk is attributed to genetic and prenatal environmental factors, though the environmental agents are incompletely characterized.
In Early Autism Risk Longitudinal Investigation (EARLI) and Markers of Autism Risk in Babies Learning Early Signs (MARBLES), two pregnancy cohorts of siblings of children with ASD, urinary metals concentrations during two pregnancy time periods (< 28 weeks and ≥ 28 weeks of gestation) were measured using inductively coupled plasma mass spectrometry. At age three, clinicians assessed ASD with DSM-5 criteria. In an exposure-wide association framework, using multivariable log binomial regression, we examined each metal for association with ASD status, adjusting for gestational age at urine sampling, child sex, age at pregnancy, race/ethnicity and education. We meta-analyzed across the two cohorts.
In EARLI ( n = 170) 17% of children were diagnosed with ASD, and 44% were classified as having non-neurotypical development (Non-TD). In MARBLES ( n = 231), 21% were diagnosed with ASD, and 14% classified as Non-TD. During the first and second trimester period (< 28 weeks), having cadmium concentration over the level of detection was associated with 1.69 (1.08, 2.64) times higher risk of ASD, and 1.29 (0.95, 1.75)times higher risk of Non-TD.
A doubling of first and second trimester cesium concentration was marginally associated with 1.89 (0.94, 3.80) times higher risk of ASD, and a doubling of third trimester cesium with 1.69 (0.97, 2.95) times higher risk of ASD.
Exposure in utero to elevated levels of cadmium and cesium, as measured in urine collected during pregnancy, was associated with increased risk of developing ASD.
Peer Review reports
Autism Spectrum Disorder (ASD) presents a major public health concern. ASD is a neurodevelopmental disorder characterized by impairments in social communication, social interaction, and restrictive and repetitive behavioral patterns and interests [ 1 ]. In the United States, 1 in 36 children are affected by ASD, with the prevalence among males 3.8 times greater than among females [ 2 ]. Individuals with ASD and their families face significant social and financial burdens, with higher costs for individuals with more severe ASD [ 3 ]. The social cost of ASD was greater than $7 trillion between the years 1990 – 2019 and is projected to be an additional $4 to $15 trillion by 2029 [ 4 ]. Increases in the prevalence of diagnosed ASD have been attributed to increasing social awareness [ 5 ], changes to diagnostic criteria [ 6 ], and participation in early intervention services [ 7 ]. However, the full source of this increase is largely unknown, suggesting incidence may be rising. Environmental exposures may play a role in this increase. Understanding modifiable risk factors for ASD could play a major role in guiding public health interventions.
Metals exposures are potential modifiable risk factors in ASD. Among children diagnosed with ASD relative to controls, higher childhood blood levels of arsenic [ 8 ], mercury [ 8 , 9 , 10 ], lead [ 10 , 11 , 12 ], and cadmium [ 13 ] have been observed. Although these findings are suggestive, exposure to metals was measured after ASD diagnosis, and it is not known if elevated exposure levels preceded ASD. Studies examining metals exposure during pregnancy and ASD diagnosis are less common. In the United States, persons of childbearing age experience widespread environmental exposure to metals, and higher concentrations have been observed in pregnant compared to non-pregnant people [ 14 , 15 ]. Important neurodevelopmental processes occur during the in utero period [ 16 ], and exposure to environmental factors such as metals are suggested to have a role in ASD etiology [ 17 , 18 , 19 ]. Poorer performance on social and behavioral tests among children at age 3 was associated with elevated manganese levels in infant toenails and arsenic in toenails of pregnant persons [ 20 ], and blood lead levels of pregnant persons [ 21 ]. In contrast, elevated copper levels in urine or blood collected during pregnancy was associated with decreased behavior problems assessed in children aged 3–7 years [ 22 ]. Additionally, elemental biomarkers measured in hair at one month of age, including essential and non-essential metals, have been shown to be predictive of future ASD diagnosis [ 23 ]. One nested case–control study in the Norwegian Mother, Father, and Child Cohort Study linked with the Norwegian Patient Registry examined blood metals concentrations during pregnancy, finding elevated arsenic, cadmium, and manganese were associated with ASD, and lower levels of cesium, copper, mercury, and zinc were associated with ASD [ 24 ]. There are limited studies on prenatal metals exposure and ASD, and more prospective cohorts with exposure measures of multiple metals are needed.
Given the previous heterogeneous inclusion of and approaches for different metals with ASD, we were motivated to perform a consistent discovery analysis across multiple metals. This study was conducted in two pregnancy cohorts of siblings of children with ASD, the Early Autism Risk Longitudinal Investigation (EARLI) and the Markers of Autism Risk in Babies—Learning Early Signs (MARBLES) study. The goal of this study was to use an environment-wide association study (ExWAS) framework to screen a panel of twenty-two metals measured during two time periods of pregnancy for associations with ASD diagnosis in children at age 3 years. The ExWAS is a data-driven exploratory approach to identify a subset of exposure measures most strongly associated with a trait [ 25 ] for follow-up in toxicologic or epidemiologic designs [ 26 ]. The design is inspired by the genome-wide association framework, and involves testing pairwise relationships between exposures and the outcome, producing interpretable results while accounting for multiple comparisons [ 25 ].
Study sample
The Early Autism Risk Longitudinal Investigation (EARLI) and Markers of Autism Risk Learning Early Signs (MARBLES) studies are prospective pregnancy cohorts to study autism etiology [ 27 , 28 ]. These studies recruited parents of children with clinically confirmed ASD (probands) who were early in a subsequent pregnancy or were trying to become pregnant. Siblings of children with ASD are more likely to have a diagnosis of ASD or other developmental delays [ 29 , 30 ]. In EARLI there was 232 participants with a subsequent child (sibling) born during the study between November 2009 and March 2012. In MARBLES there was 389 enrolled participants that gave birth to 425 subsequent children (sibling) between December 1, 2006 and July 1, 2016.
Covariate and outcome assessment
Demographics, pregnancy behaviors, and medical history were all collected via questionnaire at enrollment. Clinicians assessed children born during the study (siblings) at age three years using DSM-5 criteria. Children were categorized into three groups: typically developing, ASD, or non-typical development. Outcome categorization, based on a previously published algorithm using the Autism Diagnostic Observation Schedule (ADOS) and the Mullen Scales of Early Learning (MSEL) [ 31 ], has been described in these cohorts previously [ 32 , 33 ]. In brief, those who met diagnostic DSM-5 criteria and ADOS scores over the cutoff were categorized in the ASD group. Those that did not meet diagnostic criteria, but had ADOS scores within three points of the cutoff or MSEL scores 1.5 to 2 standard deviations below average, were categorized in the non-typical development group. Finally, the typical development group did not meet diagnostic criteria for ASD and were not categorized in the non-typical development group.
Exposure assessment
Urinary metals measures generally reflect acute or recent exposure, with exceptions such as cadmium reflecting cumulative exposure, and some metals such as lead and manganese have unclear urinary measure relations [ 34 ]. Timeframes of exposure reflected in urinary metals measures are summarized in Table S1. Urine samples were collected at two time periods during pregnancy: trimester 1/ trimester 2 (T1/T2) collected between 5 to less than 28 weeks of pregnancy (approximate mean 19 weeks), and trimester 3 (T3) collected at 28 to 40 weeks of pregnancy (approximate mean 32 weeks). Urinary concentrations of a panel of metals were measured using inductively coupled plasma mass spectrometry by NSF International (Centers for Disease Control and Prevention method 3018.3, with modifications for the expanded metals panel and the Thermo Scientific iCAP RQ instrument). Metals measured include antimony, arsenic, barium, beryllium, cadmium, cesium, chromium, cobalt, copper, lead, manganese, mercury, molybdenum, nickel, platinum, selenium, thallium, tin, tungsten, uranium, vanadium, and zinc. Samples for both cohorts were randomized together into two laboratory runs and runs had variable limits of detection (LOD) (Table S2). To assess urinary dilution, specific gravity was measured by NSF International using an ATAGO handheld digital refractometer model PAL-10S.
Used in a sensitivity analysis, blood concentrations during pregnancy of cadmium, manganese, lead, selenium, and total mercury were also measured in EARLI. Venous blood samples were collected from pregnant participants in trace metal free EDTA tubes. Blood samples from the first study visit ( n = 215) were used. Metal concentrations in blood samples were measured by inductively coupled dynamic reaction cell plasma mass spectrometry by the US Centers for Disease Control and Prevention (ELAN DRC II, PerkinElmer Norwalk, CT) (method DLS 3016.8, Centers for Disease Control and Prevention). Micro-clotting of the archived blood prevented measures in half of samples, leaving n = 92 with measured concentrations, as well as full data on outcome and covariates.
Statistical analyses
We used R statistical software (version 4.0.2) for statistical analysis. Code to produce analyses is available ( https://github.com/bakulskilab /Urine-Metals-ASD). The percentage of samples above LOD were computed with all samples (regardless of pregnancy time period or cohort) pooled together. Metals with less than approximately 10% of samples above the LOD were dropped from analysis (beryllium, platinum, tungsten, uranium, vanadium). Metals with less than 75% of samples above the LOD (antimony, cadmium, chromium, lead) were treated as binary variables, based on whether a sample was above or below the LOD. For the rest of the metals, concentrations were used as continuous variables. We substituted all urinary metals measures quantitated with values below the LOD with the value of the LOD/square root of two [ 35 ]. Metal concentrations were adjusted for specific gravity by multiplying concentrations by the ratio of [the median specific gravity – 1] and [sample specific gravity – 1] [ 36 ]. We then log 2 transformed the adjusted concentrations. Outlier metals concentrations > 5 standard deviations from the mean were dropped from analyses. The number of samples dropped per metal are listed in Table S3.
We separated urinary measures into T1/T2 and T3 pregnancy time periods. Samples with gestational age at collection < 28 weeks were considered T1/T2, and samples with gestational age at collection ≥ 28 weeks were considered T3. For those with two measures categorized in the same time period, the sample with gestational age at collection furthest from 28 weeks was used (lowest gestational age for T1/T2, highest gestational age for T3). Distribution of gestational age at urine sample collection are shown in Figure S1. We compared exposure levels in T1/T2 and T3 pregnancy with Spearman correlation tests.
We applied several exclusion criteria, summarized in Figure S2. We excluded individuals involved in a multiple birth ( n = 7 in EARLI, n = 5 in MARBLES), related siblings from non-multiple births (selecting one randomly to keep, n = 11 individuals), and those smoking during pregnancy ( n = 7 in EARLI, n = 9 in MARBLES). We excluded samples missing gestational age at collection (3 samples, from individuals that still had a valid sample from different time period), and sample closest to 28 weeks of pregnancy if two were collected during same time period (only in EARLI, 12 samples in T1/T2, 10 samples in T3) EARLI had 137 mothers with metals measures from two time periods, and 33 mothers with a measure from one time period. MARBLES had 142 mothers with metals measures from two time period, and 89 mothers with a measure from one time period. In EARLI, 3 individuals were missing education information, resulting in n = 151 for the early pregnancy period, and n = 156 for the late pregnancy period. In MARBLES, 11 individuals were missing ASD status, resulting in n = 151 for the early pregnancy period, and n = 222 for the late pregnancy period.
We calculated univariate descriptive statistics on each cohort using mean and standard deviation for continuous variables and count and frequency for categorical variables. The distributions of metal concentrations were described using mean, median, standard deviation, interquartile range, and the number and percent above the limit of detection. We calculated Spearman correlation of metals concentrations within each cohort. Separately for each cohort, we compared the bivariate sample characteristics by neurodevelopmental outcome (ASD, non-typically developing, typically developing) using ANOVA tests for continuous variables and chi-square tests for categorical variables.
Models included individuals with non-missing data on urinary metal concentration, ASD status, and covariates of interest. To estimate the adjusted associations between urinary metals concentration in pregnancy and neurodevelopmental status, we used log binomial models to estimate risk ratios (RR). Due to convergence issues, we used the delta-method normal approximations for fitting models using the epitools package [ 37 ]. In the ExWAS framework, we estimated the association of each metal (in separate models) with ASD status relative to the typically developing group using the log 2 transformed concentrations, adjusting for gestational age at urine sampling, child sex, and the following characteristics of pregnant participants: age, education, self-reported race/ethnicity. We also tested metals associations with non-typically developing status (typically developing as reference) in separate log binomial models.
Models were fit separately for each cohort, then meta analyzed together using the inverse variance method in the R meta package [ 38 ]. We reported risk ratios and 95% confidence intervals (95% CI) for each association and visualized the results using forest plots. For metals that were modeled continuously, since concentrations were log 2 -transformed, the reported associations are for a doubling in concentration. For metals that were modeled as binary, we reported the RR for above versus below the LOD. To account for multiple comparisons, we also reported false discovery rate adjusted p-values.
We performed several sensitivity analyses to assess the robustness of our findings. Since runs for metals measures had variable LODs, which impacts binary categorization and imputation for values below LOD, we ran models adjusted for batch. We also performed multivariable logistic regression for each of our models to generate adjusted odds ratios (OR) that may be compared to the risk ratios and to prior findings in the literature. Lastly, we performed analyses on the subset of EARLI samples with blood metals measures during pregnancy available and compared the findings to the findings in urinary metals.
Sample descriptive statistics
At the T1/T2 time period, urinary metal concentrations were above the LOD in greater than 75% of the samples for 13 metals in each cohort (arsenic, barium, cesium, cobalt, copper, manganese, mercury, molybdenum, nickel, selenium, thallium, tin, and zinc) (Table S4). In both EARLI and MARBLES, cobalt (Co) and nickel (Ni) concentrations had the strongest correlation (Spearman r = 0.57 in EARLI, r = 0.59 in MARBLES) (Fig. 1 ). At the T3 pregnancy time period, urinary metal concentrations were above the limit of detection in slightly less than 75% of the sample for manganese, mercury, and tin (Table S5), however they were modelled as continuous as decisions were based on LOD percentages from all samples pooled. In T3 pregnancy, cobalt and nickel remained the strongest correlated metals in MARBLES (Spearman r = 0.74), but not in EARLI. In both cohorts, lead and copper ( r = 0.43 in EARLI, r = 0.49in MARBLES) as well as lead and manganese ( r = 0.45 in both) were correlated (Figure S3).

Spearman correlations of urinary metals concentrations, measured during trimester 1 or 2 pregnancy, stratified by cohort. The upper right triangle shows the EARLI cohort. The lower left triangle shows the MARBLES cohort. Metals are represented by their chemical symbol along the diagonal
In EARLI, education and child sex assigned at birth were associated with child neurodevelopmental status. The typically developing group were birthed from individuals with higher levels of education (72% with college degree), compared to the non-typically developing (54%) and ASD groups (45%). The typically developing and non-typically developing groups had a similar proportion of males (43% and 49%) but lower proportion than the ASD group (83%). In MARBLES, compared to the typically developing group (52% male) and the non-typically developing group (55% male), ASD (67% male) had higher proportion of males (Table 1 ). In the T1/T2 pregnancy time period there were metals concentrations available from 151 urine samples in EARLI (63 typically developing, 67 non-typically developing, 21 ASD) and 151 in MARBLES (100 typically developing, 17 non-typically developing, 34 ASD) (Table S6). At the T3 pregnancy time period, there were 156 samples with urinary metal concentrations available in EARLI (63 typically developing, 68 non-typically developing, 25 ASD) and 222 in MARBLES (141 typically developing, 33 non-typically developing, 48 ASD) (Table S7).
For participants with two time periods, correlation between the two were strongest for measured cesium ( r = 0.53 in EARLI, r = 0.58 in MARBLES), mercury ( r = 0.50 in EARLI, r = 0.43 in MARBLES), tin ( r = 0.59 in EARLI, r = 0.53in MARBLES), and zinc ( r = 0.56 in EARLI, r = 0.51 in MARBLES). Cross time period correlation was weakest for cobalt ( r = 019 in EARLI, r = 0.30 in MARBLES), manganese ( r = 0.12 in EARLI, r = -0.07 in MARBLES), and molybdenum ( r = 0.3 in EARLI, r = 0.09 in MARBLES) (Table S8).
Urinary metal association with autism spectrum disorder status
We examined associations between urinary metals in the T1/T2 pregnancy time period and ASD. In meta-analysis, comparing ASD to typical development, having urine cadmium concentration above the limit of detection was associated with 1.69 (95% CI 1.08, 2.64) times higher risk for ASD (EARLI RR = 1.85, 95% CI 0.90, 3.81; MARBLES RR = 1.60, 95% CI 0.91, 2.82). (Fig. 2 , Table S9). A doubling in arsenic was associated with lower ASD risk (RR = 0.84, 95% CI 0.74, 0.94), driven by the EARLI cohort (EARLI RR = 0.80, 95% CI 0.69, 0.91; MARBLES RR = 1.0, 95% CI 0.77, 1.29). Similarly, selenium was associated with lower ASD risk (RR = 0.89, 95% CI 0.83,0.95), driven by the MARBLES cohort (EARLI RR = 1.46, 95% CI 0.37, 5.75; MARBLES RR = 0.88, 95% CI 0.83, 0.94). Thallium concentration doubling was associated with RR = 1.16 (95% CI 1.05, 1.28), with stronger effect in MARBLES (RR = 1.17, 95% CI 1.06, 1.30) than in EARLI (RR = 1.02, 95% CI 0.65, 1.61). Marginal associations were observed with cesium, where a doubling in urinary concentration was estimated to have RR = 1.89 (95% CI 0.94, 3.8). The associations for arsenic (FDR = 0.05), selenium (FDR = 0.01), and thallium (FDR = 0.05) reached FDR < 0.1 when adjusting for multiple comparisons. No associations were observed between the remaining urinary metal concentrations and ASD status at the T1/T2 pregnancy time period.

Adjusted risk ratios for the associations between urinary metals concentrations measuring during pregnancy and risk of autism spectrum disorder, relative to typically developing. Antimony, cadmium, chromium, and lead compare over limit of detection vs under the limit of detection for that metal. Remaining metals show the risk ratio for a doubling in metal concentration. Analyses were performed stratified by cohort (EARLI and MARBLES) and then meta-analyzed across cohorts. Red/diamond denotes a nominal meta-analysis p -value < 0.05, and orange/square a nominal meta-analysis p -value < 0.10
At the T3 pregnancy time period, we estimated the association between each metal concentration and ASD. Comparing ASD to typically developing in meta-analyses, a doubling in cesium was marginally associated with ASD (RR = 1.69, 95% CI 0.97, 2.95) in meta-analysis (EARLI RR = 1.77, 95% CI 0.70, 4.46.; MARBLES RR = 1.65, 95% CI 0.83, 3.31) (Fig. 2 , Table S9). A doubling in thallium was associated with ASD with RR = 1.16 (95% CI 1.08,1.25), though effects were different between cohorts (EARLI RR = 0.87, 95% CI 0.50, 1.53.; MARBLES RR = 1.17, 95% CI 1.08, 1.26). A doubling in tin was associated with 1.13 (95% CI 1.01,1.26) times risk of ASD. The association with thallium reached FDR < 0.1. No associations were observed between the remaining urinary metal concentrations and ASD status at the T3 pregnancy time period.
Urinary metal association with non-typically developing status
We repeated the adjusted regression analyses to estimate the association of T1/T2 pregnancy urinary metals and non-typically developing status. A marginal relationship with mercury and non-typical development was observed, where a doubling in concentration had estimated RR = 1.06 (95% CI 0.99, 1.14), driven by the EARLI cohort (EARLI RR = 1.07, 95% CI 1.00, 1.15; MARBLES RR = 0.73, 95% CI 0.46, 1.18). A doubling of nickel was marginally associated with 1.391.40 (95% CI 0.99, 1.96) times risk of non-typical development, also driven by the EARLI cohort (EARLI RR = 1.56, 95% CI 1.06, 2.30; MARBLES RR = 0.91, 95% CI 0.43, 1.93) (Fig. 3 ,Table S10). Though not statistically significant, having urine cadmium concentrations above the LOD was suggestive of elevated risk of non-typical development, with RR = 1.29 (95% CI 0.95, 1.75), and a doubling of cesium was suggestive of elevated non-typical development risk (RR = 1.49, 95% CI 0.90, 2.49). No associations were observed between the remaining urinary metal concentrations and non-typically developing status at the T1/T2 pregnancy time period.

Adjusted risk ratios for the associations between urinary metals concentrations measuring during pregnancy and risk of non-typically developing, relative to typically developing. Antimony, cadmium, chromium, and lead compare over limit of detection vs under the limit of detection for that metal. Remaining metals show risk ratio for a doubling in metal concentration. Analyses were performed stratified by cohort (EARLI and MARBLES) and then meta-analyzed across cohorts. Red/diamond denotes a nominal meta-analysis p -value < 0.05, and orange/square a nominal meta-analysis p -value < 0.10
We examined associations between non-typically developing and the T3 pregnancy metals measures. (Fig. 3 , Table S10). A doubling of the essential metal selenium concentration was associated in meta-analysis with 0.87 (95% CI 0.76, 1.00) times lower risk of non-typically developing status, driven by precision of results in MARBLES and had opposite directions of effect by cohort (EARLI RR = 1.20, 95% CI 0.61, 2.36; MARBLES RR = 0.86, 95% CI 0.75, 0.99). A doubling of the essential metal zinc concentration was associated with 0.97 (95% CI 0.94, 0.99) times lower risk of non-typically developing status. No associations were observed between the remaining urinary metal concentrations and non-typically developing status at the T3 pregnancy time period.
Pregnancy blood metal association with neurodevelopmental status
In EARLI, 92 blood samples collected during pregnancy had available covariate and blood metals measures (41 typically developing, 32 non- typically developing, 19 ASD) (Table S11). A doubling in blood cadmium was marginally associated with 1.11 (95% CI 0.96, 1.29) times higher risk of ASD, and a doubling in blood lead was associated with 1.23 (95% CI 1.01, 1.54) times higher risk of ASD (Figure S4). A doubling in cadmium was also associated with 1.10 (95% CI 1.02, 1.19) times higher risk of non-typical development. A doubling in blood lead was associated with 1.16 (95% CI 1.00, 1.35) times higher risk of non-typical development (Figure S4). No associations were observed between the remaining blood metal concentrations (mercury, selenium, manganese) and neurodevelopmental status.
Sensitivity analysis
With batch as a covariate (Tables S12 and S13), the cadmium association in T1/T2 pregnancy with ASD remained consistent where being over the limit of detection was associated with 1.68 (95% CI 1.08, 2.62) times higher risk of ASD. With batch adjustment, antimony in T1/T2 pregnancy was marginally associated with ASD, with estimated RR = 1.64 (95% CI 0.98, 2.73). The estimated associations between cesium and ASD had consistent magnitude with slight attenuation. On the other hand, the relationships with thallium and tin with ASD were attenuated.
Using logistic regression models, consistency to the previous log binomial findings was observed for cadmium and cesium. In general, estimates on the odds ratio scale were higher in magnitude and significance for cadmium and cesium. In particular, the T1/T2 cadmium association with non-typically developing was stronger in logistic regression, with an estimated OR = 1.95 (95% CI 1.05, 3.63). Strength of relationships between T1/T2 arsenic, thallium, and tin with ASD were attenuated with larger confidence intervals, while T3 arsenic showed marginal association with ASD (OR = 1.21, 95% CI 0.97, 1.49) when using logistic regression (Table S14) . T1/T2 pregnancy molybdenum and nickel associations with non-typical development strengthened in logistic regression, while selenium, zinc, and mercury associations with non-typical development were attenuated (Table S15) .
In two prospective birth cohorts of siblings of children with ASD, we measured urinary metals levels during two pregnancy time periods and examined relationships to ASD or non-typical development status at age 3 in an ExWAS framework. Our most consistent finding was heightened risk of atypical neurodevelopment related to T1/T2 cadmium exposure. Although the relationships were not significant in T3 pregnancy, the directions of effect were consistent across time periods. Furthermore, similar findings were observed in the blood subsample. Cesium related to atypical neurodevelopment was also notable, with consistency across ASD and non-typical development outcomes and time period, with exception of T3 pregnancy cesium and non-typical development. Cadmium and cesium associations were also the most robust to different modelling strategies. This study suggests metals exposure during pregnancy may be related to risk of ASD or non-typical development status at age 3. Existing studies have also examined the relationship between metals exposure and ASD with considerable heterogeneity in exposure timing and matrices measured [ 39 ].
Our findings with cadmium align with previous results, while cesium differed. The study in the Norwegian Mother, Father, and Child Cohort Study found higher odds of ASD for children in the highest quartile of cadmium exposure measured in blood during pregnancy [ 24 ], matching results from the present study. The same study found the highest quartile of pregnancy blood cesium levels had lower odds of ASD compared to the lowest quartile, while in contrast our study suggests higher risk of ASD with higher urinary cesium. Cadmium exposure can occur through release into the environment by industrial processes such as smelting and battery production, and subsequent bioaccumulation in plants, including tobacco [ 34 ]. Thus, diet and smoking may play a role in observed associations. Though our analysis was restricted to those who did not smoke during pregnancy, exposure through second-hand smoke is possible. Furthermore, cadmium exposure can occur though air pollution [ 34 ], and air pollution during pregnancy has been associated with ASD [ 40 , 41 ]. Cadmium exposure is associated with adverse pregnancy and birth outcomes, and can accumulate in the placenta [ 42 ]. Possible mechanisms of health impacts of cadmium include impacting gap junction formation in embryo development, oxidative stress in the placenta, disruption of nutrient homeostasis in the placenta, and aberrant DNA methylation and subsequent gene expression [ 42 , 43 ]. Cesium has been detected in ash from coal power plants and hazardous waste incineration, and can be absorbed in plants after deposition, with most human exposure occurring through diet [ 44 ]. Cesium has been related to birth outcomes such as small for gestational age [ 45 ]. Correlation between cesium and thallium may have contributed to observed associations between thallium and ASD, driven primarily by MARBLES. In MARBLES, thallium and cesium were correlated (Spearman r = 0.45), while in EARLI where thallium associations were not observed the two metals were only moderately correlated (Spearman r = 0.23). However, thallium has been found to at higher levels in urine of individuals with ASD compared to neurotypical controls [ 46 ], and has been related to pre-term birth with proposed mechanisms of toxicity including ability to cross placenta and oxidative stress [ 47 ].
Lead is a known neurotoxicant, and exposure matrix was an important factor. A systematic review and meta-analysis of lead concentrations in children with ASD from cross-sectional and case–control studies showed significant difference in child blood lead levels (samples collected in ASD and control group children at mean age 6.91 and 6.74 years respectively) compared to controls, but not in child urinary lead levels (samples collected in ASD and control group children at mean age 8.64 and 8.47 years respectively) [ 48 ] . This mirrors our results in measures during pregnancy, where we found blood lead levels of pregnant individuals were associated with risk of ASD or non-typical development in offspring, but not pregnancy urinary lead levels. Blood lead is a more reliable measure of recent exposure compared to urinary or hair lead levels [ 49 ], which may explain our findings of stronger blood lead ASD associations than those seen with urinary lead.
Our results for selenium and arsenic were mixed. A doubling of T3 pregnancy selenium concentration was associated with lower risk of non-typical development, however there were opposite effect estimates between cohorts. Selenium supplementation in an animal model attenuated autism phenotype [ 50 ], and studies measuring selenium cross-sectionally in children in Saudi Arabia [ 51 ] and China [ 52 ] found lower selenium levels in those with ASD. On the other hand, two-sample Mendelian randomization analysis using genetic instruments of blood and blood-toenail selenium suggest selenium levels are associated with increased risk of ASD [ 53 ], and in the Boston Birth Cohort red blood cell selenium levels measured during pregnancy at near delivery were associated with increased odds of ASD in children [ 54 ]. Selenium’s main route of exposure is through diet containing selenium rich foods or plants/animals that have bioaccumulated selenium from industrial or agricultural release [ 34 ]. Thus, findings and cohort differences may be due to uncorrected confounding from impact of diet during pregnancy. We found T1/T2 arsenic to be protective for ASD, but only in the EARLI cohort. In contrast, higher arsenic in blood during pregnancy has previously been seen to be associated with increased ASD [ 24 ]. Urinary arsenic measures both organic and inorganic arsenic, which can be impacted by seafood consumption [ 34 ]. Additionally, erythrocyte levels of arsenic have been seen to increase with fresh fruit consumption [ 55 ]. Urinary arsenic measures and associations in this and other studies may be confounded by dietary patterns, which may differ between cohorts.
Our findings add to a growing body of evidence of the neurodevelopmental impacts of metals exposure during pregnancy, and has several strengths. We were able to assay a wide array of metals with high detection rates in two different birth cohorts, at two different time periods. In one cohort, we were also able to evaluate five metals in a different exposure matrix: blood during pregnancy. The longitudinal design allowed examination of exposure measures during pregnancy that preceded subsequent ASD outcome three years after birth. The enriched risk cohort design ensured all participants were clinically assessed using gold standards for ASD diagnosis.
We used the ExWAS framework to consistently perform evaluations across metals, time periods, and cohorts. This approach has been effectively used with other outcomes to screen exposures for further testing with laboratory and population based approaches [ 25 , 26 ]. Our hypothesis generating findings prioritize cadmium and cesium for examination with ASD. In the ExWAS approach, we considered each metal individually. This is complementary to mixtures approaches, which consider combinations of metals together [ 56 ]. Mixtures approaches offer important ways to account for correlations and interactions among exposures. In these same cohorts, mixtures of metals were tested with a continuous neurodevelopmental outcome measure using Bayesian kernel machine regression [ 57 ]. Combinations of exposures to lead, mercury, selenium and manganese had inconsistent associations with Social Responsiveness Score at age three across the EARLI and MARBLES cohorts [ 57 ]. ExWAS and mixtures approaches both provide useful and distinct information about the complex ways exposures can be related to health.
Our study has several limitations, which may point to areas of future research. This study modeled metals as linear or dichotomous, but some metals, especially essential nutrients, may have non-linear relationships. While the sibling cohort design allowed for an extensively phenotyped sample, our findings may not be generalizable to populations where ASD is less common, thus it would be important to also compare to results found in population-based samples. Genetic factors contributing to probability of ASD will likely be enriched in our study population, which recruited families who already had a child with ASD. Since disrupted elemental metabolism has been seen in relation to ASD [ 23 ], unaccounted genetic factors impacting metals metabolism may confound associations. Additionally, future studies should consider other exposure matrices or time periods. The choice of exposure matrix is important for exposure timing. For example, blood cadmium levels reflect recent exposure, while urinary cadmium reflects a longer, cumulative exposure [ 58 ]. Certain exposure matrices may be more reliable for some metals. Since urine samples for analysis were only available for a subset of cohort participants, selection bias may impact our results if availability or willingness to provide urine samples is related to behaviors associated with metals exposures. Our blood sample was further restricted, and selection bias may exist, especially if micro-clotting was associated with metals exposures. Future analyses in large and prospective cohorts may increase the generalizability of results.
This study suggests that prenatal exposure to toxic metals, such as cadmium, is associated with risk of ASD or non-typical development in offspring. Potential routes of exposure to metals include contamination of soil and water, through ambient air, and through use in industrial applications or domestic products [ 59 ]. Further studies determining the population attributable risk from metals exposures and establishment of causality are necessary to evaluate whether public health measures to reduce these exposures during pregnancy can be used as preventative strategy for neurodevelopmental disorders.
Availability of data and materials
Data used in this manuscript is publicly available through the National Institute of Mental Health Data Archive (EARLI cohort repository: 1600, MARBLES cohort repository: 1946, EARLI/MARBLES metals repository: 2462) and through data requests to the Principal Investigators of cohorts (EARLI: MDF, MARBLES: RJS).
Abbreviations
- Autism spectrum disorder
Early autism risk longitudinal investigation
Markers of autism risk in babies - learning early signs
Autism diagnostic observation schedule
Mullen scales of early learning
Limit of detection
Trimester 1
Trimester 2
Trimester 3
Exposure-wide association study
American Psychiatric Association. APA Publishing information: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders : DSM-5. 5th ed. American Psychiatric Association Publishing; 2013.
Maenner MJ, Warren Z, Williams AR, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72(2):1–14. https://doi.org/10.15585/mmwr.ss7202a1 .
Article Google Scholar
Rogge N, Janssen J. The economic costs of autism spectrum disorder: a literature review. J Autism Dev Disord. 2019;49(7):2873–900. https://doi.org/10.1007/s10803-019-04014-z .
Cakir J, Frye RE, Walker SJ. The lifetime social cost of autism: 1990–2029. Res Autism Spectr Disord. 2020;72(January):1–18. https://doi.org/10.1016/j.rasd.2019.101502 .
Keyes KM, Susser E, Cheslack-postava K, Fountain C, Liu K, Bearman PS. Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in California. Int J Epidemiol. 2012;41(2):495–503. https://doi.org/10.1093/ije/dyr193 .
Hansen SN, Schendel DE, Parner ET. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 2015;169(1):56–62. https://doi.org/10.1001/jamapediatrics.2014.1893 .
Worley JA, Matson JL, Sipes M, Kozlowski AM. Prevalence of autism spectrum disorders in toddlers receiving early intervention services. Res Autism Spectr Disord. 2011;5(2):920–5. https://doi.org/10.1016/j.rasd.2010.10.007 .
Ding M, Shi S, Qie S, Li J, Xi X. Association between heavy metals exposure (cadmium, lead, arsenic, mercury) and child autistic disorder: a systematic review and meta-analysis. Front Pediatr. 2023;11:1169733. https://doi.org/10.3389/fped.2023.1169733
Jafari T, Rostampour N, Fallah AA, Hesami A. The association between mercury levels and autism spectrum disorders: a systematic review and meta-analysis. J Trace Elem Med Biol. 2017;44(May):289–97. https://doi.org/10.1016/j.jtemb.2017.09.002 .
Article CAS Google Scholar
Zhang J, Li X, Shen L, et al. Trace elements in children with autism spectrum disorder : a meta-analysis based on case-control studies. J Trace Elem Med Biol. 2021;67(May):126782.
Saghazadeh A, Rezaei N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(July):340–68. https://doi.org/10.1016/j.pnpbp.2017.07.011 .
Rashaid AHB, Nusair SD, Alqhazo MT, Adams JB, Abu-Dalo MA, Bashtawi MA. Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: a case-control study on Jordanian children. J Trace Elem Med Biol. 2021;67(May). https://doi.org/10.1016/j.jtemb.2021.126790 .
Baj J, Flieger W, Flieger M, et al. Autism spectrum disorder: trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci Biobehav Rev. 2021;129(July):117–32. https://doi.org/10.1016/j.neubiorev.2021.07.029 .
Martin EM, Fry RC. Environmental influences on the epigenome: exposure- associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–33. https://doi.org/10.1146/annurev-publhealth-040617-014629 .
Watson CV, Lewin M, Ragin-Wilson A, et al. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ Res. 2020;183. https://doi.org/10.1016/j.envres.2020.109208 .
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353(6301):772–7. https://doi.org/10.1126/science.aag3194 .
Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–97. https://doi.org/10.1007/s00018-018-2988-4 .
Heyer DB, Meredith RM. Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology. 2017;58:23–41. https://doi.org/10.1016/j.neuro.2016.10.017 .
Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443–64. https://doi.org/10.1093/ije/dyt282 .
Doherty BT, Romano ME, Gui J, et al. Periconceptional and prenatal exposure to metal mixtures in relation to behavioral development at 3 years of age. Environ Epidemiol. 2020;4(4). https://doi.org/10.1097/EE9.0000000000000106 .
Fruh V, Rifas-Shiman SL, Amarasiriwardena C, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019;75(May):105–15. https://doi.org/10.1016/j.neuro.2019.09.006 .
Jedynak P, Maitre L, Guxens M, et al. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age – an exposome-based approach in 5 European cohorts. Sci Total Environ. 2021;763(December 2020):144115. https://doi.org/10.1016/j.scitotenv.2020
Austin C, Curtin P, Arora M, et al. Elemental dynamics in hair accurately predict future autism spectrum disorder diagnosis: an international multi-center study. J Clin Med. 2022;11(23):7154. https://doi.org/10.3390/jcm11237154
Skogheim TS, Weyde KVF, Engel SM, et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ Int. 2021;152(October 2020):106468.
Chung MK, House JS, Akhtari FS, et al. Decoding the exposome: data science methodologies and implications in exposome-wide association studies (ExWASs). Exposome. 2024;4(1). https://doi.org/10.1093/exposome/osae001
Zheng Y, Chen Z, Pearson T, Zhao J, Hu H, Prosperi M. Design and methodology challenges of environment-wide association studies: a systematic review. Environ Res. 2020;183:109275. https://doi.org/10.1016/j.envres.2020.109275
Newschaffer CJ, Croen LA, Fallin MD, et al. Infant siblings and the investigation of autism risk factors. J Neurodev Disord. 2012;4(1):7. https://doi.org/10.1186/1866-1955-4-7
Hertz-Picciotto I, Schmidt RJ, Walker CK, et al. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: design, protocols, and preliminary data from the MARBLES study. Environ Health Perspect. 126(11):117004. https://doi.org/10.1289/EHP535 .
Hansen SN, Schendel DE, Francis RW, et al. Recurrence risk of autism in siblings and cousins: a multi-national, population-based study. J Am Acad Child Adolesc Psychiatry. 2019;58(9):866–75. https://doi.org/10.1016/j.jaac.2018.11.017 .
Miller M, Musser ED, Young GS, Olson B, Steiner RD, Nigg JT. Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatr. 2019;173(2):147–52. https://doi.org/10.1001/jamapediatrics.2018.4076 .
Ozonoff S, Young GS, Belding A, et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry. 2014;53(4):398-407.e2. https://doi.org/10.1016/j.jaac.2013.12.020 .
Mordaunt CE, Park BY, Bakulski KM, et al. A meta-analysis of two high-risk prospective cohort studies reveals autism-specific transcriptional changes to chromatin, autoimmune, and environmental response genes in umbilical cord blood. Mol Autism. 2019;10:36. https://doi.org/10.1186/s13229-019-0287-z .
Philippat C, Barkoski J, Tancredi DJ, et al. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int J Hyg Environ Health. 2018;221(3):548–55. https://doi.org/10.1016/j.ijheh.2018.02.004 .
Martinez-Morata I, Sobel M, Tellez-Plaza M, Navas-Acien A, Howe CG, Sanchez TR. A state-of-the-science review on metal biomarkers. Curr Environ Health Rep. 2023;10(3):215–49. https://doi.org/10.1007/s40572-023-00402-x .
Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. https://doi.org/10.1080/1047322X.1990.10389587 .
Middleton DRS, Watts MJ, Polya DA. A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ Int. 2019;130:104721. https://doi.org/10.1016/j.envint.2019.03.069
Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–70. https://doi.org/10.1093/ije/dyu029 .
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. https://doi.org/10.1136/ebmental-2019-300117 .
Campbell KA, Hickman R, Fallin MD, Bakulski KM. Prenatal exposure to metals and autism spectrum disorder: current status and future directions. Curr Opin Toxicol. 2021;26:39–48. https://doi.org/10.1016/j.cotox.2021.04.001 .
Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr Environ Health Rep. 2015;2(4):430–9. https://doi.org/10.1007/s40572-015-0073-9 .
Yu X, Mostafijur Rahman M, Carter SA, et al. Prenatal air pollution, maternal immune activation, and autism spectrum disorder. Environ Int. 2023;179:108148. https://doi.org/10.1016/j.envint.2023.108148
Amegah AK, Sewor C, Jaakkola JJK. Cadmium exposure and risk of adverse pregnancy and birth outcomes: a systematic review and dose–response meta-analysis of cohort and cohort-based case–control studies. J Expo Sci Environ Epidemiol. 2021;31(2):299–317. https://doi.org/10.1038/s41370-021-00289-6 .
Geng HX, Wang L. Cadmium: toxic effects on placental and embryonic development. Environ Toxicol Pharmacol. 2019;67:102–7. https://doi.org/10.1016/j.etap.2019.02.006 .
ATSDR. Toxicological Profile for Cesium. Atlanta: Agency for Toxic Substances and Disease Registry (US); 2004.
Issah I, Duah MS, Arko-Mensah J, Bawua SA, Agyekum TP, Fobil JN. Exposure to metal mixtures and adverse pregnancy and birth outcomes: a systematic review. Sci Total Environ. 2024;908:168380. https://doi.org/10.1016/j.scitotenv.2023.168380
Adams J, Howsmon DP, Kruger U, et al. Significant association of urinary toxic metals and autism-related symptoms—a nonlinear statistical analysis with cross validation. PLoS One. 2017;12(1):e0169526. https://doi.org/10.1371/journal.pone.0169526
Chen X, Huang L, Li Q, et al. Effect of maternal thallium exposure in early pregnancy on the risk of preterm birth. Environ Sci Pollut Res. 2022;29(33):49966–75. https://doi.org/10.1007/s11356-022-19332-6 .
Nakhaee S, Amirabadizadeh A, Farnia V, Ali Azadi N, Mansouri B, Radmehr F. Association between biological lead concentrations and Autism Spectrum Disorder (ASD) in children: a systematic review and meta-analysis. Biol Trace Elem Res. 2023;201(4):1567–81. https://doi.org/10.1007/s12011-022-03265-9 .
ATSDR. Toxicological Profile for Lead. Atlanta: Agency for Toxic Substances and Disease Registry (US); 2020.
Wu H, Zhao G, Liu S, et al. Supplementation with selenium attenuates autism-like behaviors and improves oxidative stress, inflammation and related gene expression in an autism disease model. J Nutr Biochem. 2022;107:109034. https://doi.org/10.1016/j.jnutbio.2022.109034
El-Ansary A, Bjørklund G, Tinkov AA, Skalny AV, Al DH. Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis. 2017;32(4):1073–80. https://doi.org/10.1007/s11011-017-9996-1 .
Wu J, Wang D, Yan L, et al. Associations of essential element serum concentrations with autism spectrum disorder. Environ Sci Pollut Res Int. 2022;29(59):88962–71. https://doi.org/10.1007/s11356-022-21978-1 .
Guo X, Tang P, Hou C, Li R. Mendelian randomization investigation highlights different roles of selenium status in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2023;122:110694. https://doi.org/10.1016/j.pnpbp.2022.110694
Lee ASE, Ji Y, Raghavan R, et al. Maternal prenatal selenium levels and child risk of neurodevelopmental disorders: a prospective birth cohort study. Autism Res. 2021;14(12):2533–43. https://doi.org/10.1002/aur.2617 .
Lin PD, Cardenas A, Rifas-Shiman SL, et al. Diet and erythrocyte metal concentrations in early pregnancy—cross-sectional analysis in project viva. Am J Clin Nutr. 2021;114(2):540–9. https://doi.org/10.1093/ajcn/nqab088 .
Yu L, Liu W, Wang X, et al. A review of practical statistical methods used in epidemiological studies to estimate the health effects of multi-pollutant mixture. Environ Pollut. 2022;306:119356. https://doi.org/10.1016/j.envpol.2022.119356
Yu EX, Dou JF, Volk HE, et al. Prenatal metal exposures and child social responsiveness scale scores in 2 prospective studies. Environ Health Insights. 2024;18:11786302231225312. https://doi.org/10.1177/11786302231225313 .
ATSDR. Toxicological Profile for Cadmium. Atlanta: Agency for Toxic Substances and Disease Registry (US); 2012.
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metals toxicity and the environment. EXS. 2012;101:133–64. https://doi.org/10.1007/978-3-7643-8340-4_6 .
Download references
Acknowledgements
Not applicable.
Funding for the EARLI study was provided by the National Institutes of Health (R01ES016443, R24ES030893) and Autism Speaks (003953). Funding for the MARBLES study was provided by the National Institutes of Health (R01ES020392, R01ES028089, R/U24ES028533, and P01ES011269) and the United States Environmental Protection Agency Science to Achieve Results program (#RD-83329201). Funding for metals measures and this work was supported by the National Institutes of Health (R01ES025531). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
University of Michigan, Ann Arbor, MI, USA
John F. Dou, Manon M. Nitta & Kelly M. Bakulski
University of California Davis, Davis, CA, USA
Rebecca J. Schmidt & Irva Hertz-Picciotto
Johns Hopkins University, Baltimore, MD, USA
Heather E. Volk & Jason I. Feinberg
Penn State University, State College, PA, USA
Craig J. Newschaffer
Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA
Lisa A. Croen
Rollins School of Public Health, Emory University, Atlanta, GA, USA
M. Daniele Fallin
You can also search for this author in PubMed Google Scholar
Contributions
JFD analyzed data. JFD, MMN, and KMB were major contributors in writing the manuscript. RJS, HEV, JIF, CJN, LAC, IHP, and MDF made substantial contributions to the conception and design of the work, and to acquisition of data. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kelly M. Bakulski .
Ethics declarations
Ethics approval and consent to participate.
The EARLI study was reviewed and approved by Human Subjects Institutional Review Boards (IRBs) from each of the four study sites (Johns Hopkins University, Drexel University, University of California Davis, and Kaiser Permanente Northern California). The MARBLES protocol was reviewed and approved by the Human Subjects IRB from University of California Davis. Secondary data analysis for this manuscript was approved by the Human Subjects IRB for the University of Michigan. Written informed consent was obtained from the parents for collection of data and samples.
Consent for publication
Competing interests.
The authors declare they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dou, J.F., Schmidt, R.J., Volk, H.E. et al. Exposure to heavy metals in utero and autism spectrum disorder at age 3: a meta-analysis of two longitudinal cohorts of siblings of children with autism. Environ Health 23 , 62 (2024). https://doi.org/10.1186/s12940-024-01101-2
Download citation
Received : 23 January 2024
Accepted : 25 June 2024
Published : 05 July 2024
DOI : https://doi.org/10.1186/s12940-024-01101-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metals exposure
- Pregnancy cohort
- Epidemiology
Environmental Health
ISSN: 1476-069X
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Reading in Autism Spectrum Disorders: A Literature Review
Affiliation.
- 1 Department of Physical Therapy, Speech-Language Pathology and Audiology, and Occupational Therapy, School of Medicine, Universidade de Sx00E3;o Paulo (FMUSP), Sx00E3;o Paulo, Brazil.
- PMID: 26766549
- DOI: 10.1159/000442086
Objective: To review what the literature says about reading abilities of children on the autism spectrum (autism spectrum disorders, ASD) as well as to assess the results of intervention proposals. The broad ASD diagnosis used in the last decades and the resulting changes in the prevalence of these disorders have led to a relevant increase in the number of children diagnosed with ASD in the school system. The purpose of this review is to identify the different profiles of reading abilities shown by children with ASD described in the recent literature and the results of reported intervention methods.
Methods: A review of the literature was conducted in the Web of Sciences and PubMed databases with the keywords 'autism' AND 'read*' and the filter 2010-2015. All articles published in the last 5 years focusing on description of and intervention for reading abilities in individuals with ASD were included. Review articles were excluded.
Results: The selected 58 articles were divided into those that described reading abilities in individuals with ASD (n = 27) and those that reported intervention procedures for reading development (n = 31).
Conclusions: Direct comparisons and associations were prevented due to different inclusion criteria and lack of detailed information about intervention processes. We propose tentative conclusions that should be confirmed by further studies.
© 2016 S. Karger AG, Basel.
PubMed Disclaimer
Similar articles
- An evaluation of the clinical application of the DSM-5 for the diagnosis of autism spectrum disorder. Burns CO, Matson JL. Burns CO, et al. Expert Rev Neurother. 2017 Sep;17(9):909-917. doi: 10.1080/14737175.2017.1351301. Epub 2017 Jul 10. Expert Rev Neurother. 2017. PMID: 28678629 Review.
- Neuropsychological performance of Finnish and Egyptian children with autism spectrum disorder. Elsheikh S, Kuusikko-Gauffin S, Mattila ML, Jussila K, Ebeling H, Loukusa S, Omar M, Riad G, Rautio A, Moilanen I. Elsheikh S, et al. Int J Circumpolar Health. 2016 Jan 29;75:29681. doi: 10.3402/ijch.v75.29681. eCollection 2016. Int J Circumpolar Health. 2016. PMID: 26829278 Free PMC article.
- Broader Autism Phenotype in Siblings of Children with ASD--A Review. Pisula E, Ziegart-Sadowska K. Pisula E, et al. Int J Mol Sci. 2015 Jun 10;16(6):13217-58. doi: 10.3390/ijms160613217. Int J Mol Sci. 2015. PMID: 26068453 Free PMC article. Review.
- The Impact of Reading Intervention on Brain Responses Underlying Language in Children With Autism. Murdaugh DL, Deshpande HD, Kana RK. Murdaugh DL, et al. Autism Res. 2016 Jan;9(1):141-54. doi: 10.1002/aur.1503. Epub 2015 May 25. Autism Res. 2016. PMID: 26016818
- Reading comprehension, word decoding and spelling in girls with autism spectrum disorders (ASD) or attention-deficit/hyperactivity disorder (AD/HD): performance and predictors. Asberg J, Kopp S, Berg-Kelly K, Gillberg C. Asberg J, et al. Int J Lang Commun Disord. 2010 Jan-Feb;45(1):61-71. doi: 10.3109/13682820902745438. Int J Lang Commun Disord. 2010. PMID: 19343578
- Impact of reading intervention on the phonological awareness of children with autism spectrum disorder: a systematic review and meta-analysis. Santos MFPD, Aprígio LCS, Lima JVSB, Miranda FDF, Araújo CM, Taveira KVM, Salgado-Azoni CA. Santos MFPD, et al. Codas. 2024 May 13;36(3):e20220336. doi: 10.1590/2317-1782/20242022336en. eCollection 2024. Codas. 2024. PMID: 38747755 Free PMC article.
- Importance of Desensitization for Autistic Children in Dental Practice. Martínez Pérez E, Adanero Velasco A, Gómez Clemente V, Miegimolle Herrero M, Planells Del Pozo P. Martínez Pérez E, et al. Children (Basel). 2023 Apr 28;10(5):796. doi: 10.3390/children10050796. Children (Basel). 2023. PMID: 37238344 Free PMC article.
- Word reading skills in autism spectrum disorder: A systematic review. Vale AP, Fernandes C, Cardoso S. Vale AP, et al. Front Psychol. 2022 Jul 27;13:930275. doi: 10.3389/fpsyg.2022.930275. eCollection 2022. Front Psychol. 2022. PMID: 35967660 Free PMC article.
- Evoking the N400 Event-related Potential (ERP) Component Using a Publicly Available Novel Set of Sentences with Semantically Incongruent or Congruent Eggplants (Endings). Toffolo KK, Freedman EG, Foxe JJ. Toffolo KK, et al. Neuroscience. 2022 Oct 1;501:143-158. doi: 10.1016/j.neuroscience.2022.07.030. Epub 2022 Aug 11. Neuroscience. 2022. PMID: 35964833 Free PMC article.
- Recognizing Psychiatric Comorbidity With Reading Disorders. Hendren RL, Haft SL, Black JM, White NC, Hoeft F. Hendren RL, et al. Front Psychiatry. 2018 Mar 27;9:101. doi: 10.3389/fpsyt.2018.00101. eCollection 2018. Front Psychiatry. 2018. PMID: 29636707 Free PMC article. Review.
- Search in MeSH
LinkOut - more resources
Full text sources.
- S. Karger AG, Basel, Switzerland
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
What influences parental burnout in parents of children with autism spectrum disorder in China? A qualitative study
- Published: 09 July 2024
Cite this article

- Xiaohe Ren 1 ,
- Yingying Cai 1 ,
- Mengyuan Zhang 1 ,
- Yue Hou 1 ,
- Jingyi Wang 1 &
- Ou Chen ORCID: orcid.org/0000-0003-2426-8557 1
Parental burnout is associated with decreased life satisfaction of family members, increased marital conflict, and poor prognosis of children with autism spectrum disorders. Alleviating parenting burnout in parents of children with autism spectrum disorders has become a priority for many organizations worldwide in their efforts to promote recovery and provide a basis for effective interventions for caregivers.The aim of this study was to explore the experiences of parental burnout within parents of children with autism spectrum disorder in China and the factors influencing their parental burnout. A qualitative descriptive study using semi-structured interviews was conducted. The study was conducted from November 2023 to January 2024 at multiple hospital children's rehabilitation centers in Jinan, Shandong Province, China. Purposive sampling was employed to select participants. Eleven parents of children with autism spectrum disorder in China were finally interviewed. Audio-recordings were transcribed verbatim and entered into qualitative data analysis software. Qualitative data were analyzed using a content analysis approach. The interviewees' accounts suggest that there is parenting burnout in the process of raising children with ASD, which is influenced by multiple factors. This is reflected in the five themes identified during the analysis:(1) irreversible symptoms of the disease in the child; (2) distress in terms of physical and psychological factors among parents; (3) lack of external familial, social, and economic support; (4) societal discrimination; and (5) the number of children. Parenting burnout is common among parents of children with ASD. Medical staff can adjust the variable factors by paying attention to parenting burnout. To improve the psychological resilience of parents and promote positive responses to prevent and alleviate the occurrence of parenting burnout, thus improving the quality of life and adverse outcomes of parents of children with ASD.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable.
Baraskewich, J., von Ranson, K. M., McCrimmon, A., & McMorris, C. A. (2021). Feeding and eating problems in children and adolescents with autism: A scoping review. [Journal Article; Review]. Autism, 25 (6), 1505–1519. https://doi.org/10.1177/1362361321995631
Baxter, A. J., Brugha, T. S., Erskine, H. E., Scheurer, R. W., Vos, T.,... Scott, J. G. (2015). The epidemiology and global burden of autism spectrum disorders. [Journal Article; Meta-Analysis; Research Support, Non-U.S. Gov't; Review; Systematic Review]. Psychological Medicine, 45 (3), 601–613. https://doi.org/10.1017/S003329171400172X
Boccaccio, F. M., Platania, G. A., Guerrera, C. S., Varrasi, S., Privitera, C. R., Caponnetto, P.,... Castellano, S. (2023). Autism Spectrum Disorder: recommended psychodiagnostic tools for early diagnosis. [Journal Article]. Health Psychology Research, 11 , 77357. https://doi.org/10.52965/001c.77357
Borelli, J. L., Burkhart, M. L., Rasmussen, H. F., Smiley, P. A., & Hellemann, G. (2018). Children's and mothers' cardiovascular reactivity to a standardized laboratory stressor: Unique relations with maternal anxiety and overcontrol. [Journal Article]. Emotion, 18 (3), 369–385. https://doi.org/10.1037/emo0000320
Brown, M., Marsh, L., & McCann, E. (2021a). Experiences of fathers regarding the diagnosis of their child with autism spectrum disorder: A narrative review of the international research. [Journal Article; Review; Systematic Review]. Journal of Clinical Nursing, 30 (19–20), 2758–2768. https://doi.org/10.1111/jocn.15781
Brown, M., Marsh, L., & McCann, E. (2021b). Experiences of fathers regarding the diagnosis of their child with autism spectrum disorder: A narrative review of the international research. [Journal Article; Review; Systematic Review]. Journal of Clinical Nursing, 30 (19–20), 2758–2768. https://doi.org/10.1111/jocn.15781
Darling, C. A., Senatore, N., & Strachan, J. (2012). Fathers of children with disabilities: stress and life satisfaction. [Journal Article]. Stress Health, 28 (4), 269–278. https://doi.org/10.1002/smi.1427
Esposito, M., Mirizzi, P., Fadda, R., Pirollo, C., Ricciardi, O., Mazza, M.,... Valenti, M. (2023). Food selectivity in children with autism: Guidelines for assessment and clinical interventions. [Journal Article; Review]. International Journal of Environmental Research and Public Health, 20 (6). https://doi.org/10.3390/ijerph20065092
Evans, T. R., Roskam, I., Stinglhamber, F., & Mikolajczak, M. (2022). Burnout across boundaries: Can parental burnout directly or indirectly influence work outcomes? [Journal Article]. Current Psychology , 1–11. https://doi.org/10.1007/s12144-021-02687-3
Featherston, R., Barlow, J., Song, Y., Haysom, Z., Loy, B., Tufford, L.,... Shlonsky, A. (2024). Mindfulness-enhanced parenting programmes for improving the psychosocial outcomes of children (0 to 18 years) and their parents. [Journal Article; Review; Systematic Review]. Cochrane Database of Systematic Reviews, 1 (1), D12445. https://doi.org/10.1002/14651858.CD012445.pub2
Fombonne, E., Croen, L. A., Bulkley, J. E., Varga, A. M., Daida, Y. G., Hatch, B. A.,... Lynch, F. L. (2022). Emotional and behavioral problems in youth with autism: High prevalence and impact on functioning. [Journal Article; Research Support, N.I.H., Extramural]. Journal of Developmental and Behavioral Pediatrics, 43 (3), 140–148. https://doi.org/10.1097/DBP.0000000000001028
Gerain, P., & Zech, E. (2018). Does informal caregiving lead to parental burnout? Comparing parents having (or Not) children with mental and physical issues. [Journal Article]. Frontiers in Psychology, 9 , 884. https://doi.org/10.3389/fpsyg.2018.00884
Guan, J., & Li, G. (2017). Injury mortality in individuals with autism. [Journal Article; Research Support, U.S. Gov't, P.H.S.]. American Journal of Public Health, 107 (5), 791–793. https://doi.org/10.2105/AJPH.2017.303696
Ibarra-Rovillard, M. S., & Kuiper, N. A. (2011). Social support and social negativity findings in depression: perceived responsiveness to basic psychological needs. [Journal Article; Review]. Clinical Psychology Review, 31 (3), 342–352. https://doi.org/10.1016/j.cpr.2011.01.005
Kutuk, M. O., Tufan, A. E., Kilicaslan, F., Guler, G., Celik, F., Altintas, E.,... Kutuk, O. (2021). High depression symptoms and burnout levels among parents of children with autism spectrum disorders: A multi-center, cross-sectional, case-control study. [Journal Article; Multicenter Study]. Journal of Autism and Developmental Disorders, 51 (11), 4086–4099. https://doi.org/10.1007/s10803-021-04874-4
Li, Y. A., Chen, Z. J., Li, X. D., Gu, M. H., Xia, N., Gong, C.,... Huang, X. L. (2022). Epidemiology of autism spectrum disorders: Global burden of disease 2019 and bibliometric analysis of risk factors. [Journal Article]. Frontiers in Pediatrics, 10 , 972809. https://doi.org/10.3389/fped.2022.972809
Lin, G. X., Goldenberg, A., Arikan, G., Brytek-Matera, A., Czepczor-Bernat, K., Manrique-Millones, D.,... Gross, J. J. (2022). Reappraisal, social support, and parental burnout. [Journal Article]. The British Journal of Clinical Psychology, 61 (4), 1089–1102. https://doi.org/10.1111/bjc.12380
Lindstrom, C., Aman, J., & Norberg, A. L. (2011). Parental burnout in relation to sociodemographic, psychosocial and personality factors as well as disease duration and glycaemic control in children with Type 1 diabetes mellitus. [Journal Article; Research Support, Non-U.S. Gov't]. Acta Paediatrica, 100 (7), 1011–1017. https://doi.org/10.1111/j.1651-2227.2011.02198.x
Lodder, A., Papadopoulos, C., & Randhawa, G. (2020). SOLACE: A psychosocial stigma protection intervention to improve the mental health of parents of autistic children-a feasibility randomised controlled trial. [Journal Article; Randomized Controlled Trial]. Journal of Autism and Developmental Disorders, 50 (12), 4477–4491. https://doi.org/10.1007/s10803-020-04498-0
Lovell, B., & Wetherell, M. A. (2019). Affiliate stigma, perceived social support and perceived stress in caregivers of children with autism spectrum disorder: A multiple mediation study. [Journal Article]. Archives of Psychiatric Nursing, 33 (5), 31–35. https://doi.org/10.1016/j.apnu.2019.08.012
Mas, J. M., Dunst, C. J., Balcells-Balcells, A., Garcia-Ventura, S., Gine, C.,... Canadas, M. (2019). Family-centered practices and the parental well-being of young children with disabilities and developmental delay. [Journal Article]. Research in Developmental Disabilities, 94 , 103495. https://doi.org/10.1016/j.ridd.2019.103495
Mikolajczak, M., Brianda, M. E., Avalosse, H., & Roskam, I. (2018). Consequences of parental burnout: Its specific effect on child neglect and violence. [Journal Article; Multicenter Study; Research Support, Non-U.S. Gov’t]. Child Abuse and Neglect, 80 , 134–145. https://doi.org/10.1016/j.chiabu.2018.03.025
Article PubMed Google Scholar
Mikolajczak, M., & Roskam, I. (2018). A theoretical and clinical framework for parental burnout: The balance between risks and resources (BR(2)). [Journal Article]. Frontiers in Psychology , 9, 886. https://doi.org/10.3389/fpsyg.2018.00886
Rabba, A. S., Cheryl, D., & Josephine, B. (2019). Parents’ experiences of an early autism diagnosis: Insights into their needs. Research in Autism Spectrum Disorders, 66 , 101415. https://doi.org/10.1016/j.rasd.2019.101415
Ren, X., Cai, Y., Wang, J., & Chen, O. (2024). A systematic review of parental burnout and related factors among parents. [Journal Article; Research Support, Non-U.S. Gov't; Systematic Review]. BMC Public Health, 24 (1), 376. https://doi.org/10.1186/s12889-024-17829-y
Riva, C. C., Ierardi, E., Ferro, V., Gallucci, M., Parodi, C., ... Astengo, M. (2016). Mother-infant emotion regulation at three months: The role of maternal anxiety, depression and parenting stress. [Journal Article]. Psychopathology, 49 (4), 285–294. https://doi.org/10.1159/000446811
Roskam, I., Aguiar, J., Akgun, E., Arikan, G., Artavia, M., Avalosse, H.,... Mikolajczak, M. (2021). Parental burnout around the globe: A 42-country study. [Journal Article]. Affect Science, 2 (1), 58–79. https://doi.org/10.1007/s42761-020-00028-4
Roskam, I., Brianda, M. E., & Mikolajczak, M. (2018a). A step forward in the conceptualization and measurement of parental burnout: The Parental Burnout Assessment (PBA). [Journal Article]. Frontiers in Psychology, 9 , 758. https://doi.org/10.3389/fpsyg.2018.00758
Roskam, I., Raes, M. E., & Mikolajczak, M. (2017). Exhausted parents: Development and preliminary validation of the parental burnout inventory. [Journal Article]. Frontiers in Psychology, 8 , 163. https://doi.org/10.3389/fpsyg.2017.00163
Roskam, I., Raes, M. E., & Mikolajczak, M. (2018b). Corrigendum: Exhausted parents: Development and preliminary validation of the parental burnout inventory. [Published Erratum]. Frontiers in Psychology, 9 , 73. https://doi.org/10.3389/fpsyg.2018.00073
Saydam, S. Z., Cuculayef, D., Dogan, T. N., Crerand, C. E., & Ozek, M. (2021). Social experiences of turkish parents raising a child with apert syndrome: A qualitative study. [Journal Article]. Cleft Palate-Craniofacial Journal, 58 (3), 354–361. https://doi.org/10.1177/1055665620944761
Szczygiel, D., Sekulowicz, M., Kwiatkowski, P., Roskam, I., & Mikolajczak, M. (2020). Validation of the Polish version of the Parental Burnout Assessment (PBA). [Journal Article]. New Directions for Child and Adolescent Development, 2020 (174), 137–158. https://doi.org/10.1002/cad.20385
Vigouroux, S. L., & Scola, C. (2018). Differences in parental burnout: Influence of demographic factors and personality of parents and children. [Journal Article]. Frontiers in Psychology, 9 , 887. https://doi.org/10.3389/fpsyg.2018.00887
Wilson, S., & Durbin, C. E. (2010). Effects of paternal depression on fathers' parenting behaviors: a meta-analytic review. [Journal Article; Meta-Analysis]. Clinical Psychology Review, 30 (2), 167–180. https://doi.org/10.1016/j.cpr.2009.10.007
Yan, T., Hou, Y., & Deng, M. (2022). Direct, indirect, and buffering effect of social support on parental involvement among chinese parents of children with autism spectrum disorders. [Journal Article]. Journal of Autism and Developmental Disorders, 52 (7), 2911–2923. https://doi.org/10.1007/s10803-021-05170-x
Zhou, H., Xu, X., Yan, W., Zou, X., Wu, L., Luo, X., Li, T., Huang, Y., Guan, H., Chen, X., Mao, M., Xia, K., Zhang, L., Li, E., Ge, X., Zhang, L., Li, C., Zhang, X., Zhou, Y., Ding, D., … LATENT-NHC Study Team (2020). Prevalence of autism spectrum disorder in China: A nationwide multi-center population-based study among children aged 6 to 12 years. Neuroscience Bulletin, 36 (9), 961–971. https://doi.org/10.1007/s12264-020-00530-6
Download references
This work was supported by the National Natural Science Foundation of China (82172543), the Natural Science Foundation of Shandong Province (ZR2020MH006), and the Key Research and Development Program of Shandong Province (2019GSF108198), the Humanities and Social Sciences Youth Team Project of Shandong University (Grant Nos. IFYT1811, IFYT18036, and IFYT18037).
Author information
Authors and affiliations.
School of Nursing and Rehabilitation, Cheeloo College of Medicine, Shandong University, No. 44 West Culture Road, Lixia District, Box142, Jinan City, 250012, Shandong Province, China
Xiaohe Ren, Yingying Cai, Mengyuan Zhang, Yue Hou, Jingyi Wang & Ou Chen
You can also search for this author in PubMed Google Scholar
Contributions
Xiaohe Ren and Yingying Cai planned the study and designed the procedures. Yue Hou and Mengyuan Zhang analysed the study data. Jingyi Wang validated and curated data. Xiaohe Ren and Yingying Cai drafted the initial manuscript. Yue Hou and Mengyuan Zhang reviewed and revised the initial draft. Ou Chen collected patients’ and controls’ data and conducted clinical evaluations. All of the authors contributed to the final version of the manuscript and approve of its contents.
Corresponding author
Correspondence to Ou Chen .
Ethics declarations
Informed consent.
Informed consents were obtained from parents of children to be included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ren, X., Cai, Y., Zhang, M. et al. What influences parental burnout in parents of children with autism spectrum disorder in China? A qualitative study. Curr Psychol (2024). https://doi.org/10.1007/s12144-024-06301-0
Download citation
Accepted : 20 June 2024
Published : 09 July 2024
DOI : https://doi.org/10.1007/s12144-024-06301-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Autism spectrum disorder
- Parental burnout
- Qualitative research
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors. ... Demily C, Poisson A, Peyroux E, et al. Autism spectrum disorder associated with 49,XYYYY: case report and review of the literature. BMC Med Genet 2017; 18:9. 10. ...
Abstract. Autism spectrum disorder (ASD) is a set of neurodevelopmental disorders characterized by a deficit in social behaviors and nonverbal interactions such as reduced eye contact, facial expression, and body gestures in the first 3 years of life. It is not a single disorder, and it is broadly considered to be a multi-factorial disorder ...
The diagnostic criteria and treatment approaches of autism spectrum disorders (ASD) have changed greatly over the years. Currently, diagnosis is conducted mainly by observational screening tools that measure a child's social and cognitive abilities. The two main tools used in the diagnosis of ASD are DSM-5 and M-CHAT, which examine persistent deficits in interaction and social communication ...
Abstract. Importance: Autism spectrum disorder (ASD), characterized by deficits in social communication and the presence of restricted, repetitive behaviors or interests, is a neurodevelopmental disorder affecting approximately 2.3% children aged 8 years in the US and approximately 2.2% of adults. This review summarizes evidence on the ...
Abstract. Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted ...
The long-term, negative physical and mental health effects of childhood adversity are well-documented in the literature, as are the mitigating effects of resilience factors. However, for those on the autism spectrum, these phenomena are relatively unstudied and not well-understood. Articulating the concept of mental health as a function of ...
Abstract. The aetiology of autism spectrum disorder (ASD) is complex and, partly, accounted by genetic factors. Nonetheless, the genetic underpinnings of ASD are poorly defined. The presence of immune dysregulations in autistic individuals, and their families, supports a role of the immune system and its genetic regulators.
Individuals with an autism spectrum disorder (ASD) diagnosis show impairment in executive function (EF). ... Longitudinal changes in executive function in autism spectrum disorder: A systematic review and meta-analyses Autism Res ... A literature search was conducted using PubMed, PsycINFO, and Web of Science on January 29, 2024. After ...
Objective: Autism spectrum disorders (ASD) are a varying group of disorders characterized by deficiency in social interaction and restrictive patterns of behavior and interests. While there are several studies focusing on the neuro-psychiatric pathogenesis of ASD, its etiology remains unclear. The role of gut-brain-axis in ASD has been studied increasingly and a correlation between symptoms ...
This study adopted a comprehensive literature review approach, including articles from the inception of the database through July 2023, to ensure a thorough examination of the relevant research. ... The effect of physical exercise on disordered social communication in individuals with autism Spectrum disorder: a systematic review and meta ...
This paper is a literature review of some of the popular treatments for autism spectrum disorder. (ASD). It begins with an overview of ASD, the common symptoms, and comorbidities. It. includes a review of pharmacological treatments, behavioral treatments, and dietary treatments. Some treatment options in each of those categories are explained ...
Autism Spectrum Disorder (ASD) manifests as insu cient social communication, restrictive repetitive behaviors or interests, with a prevalence of around 2.3% among 8year-old children and 2.2% among ...
The findings from a systematic literature review of 24 empirical studies of interventions for post-secondary students with autism spectrum disorder (ASD) are reported in this study. A diverse range of interventions were examined, many of which appeared feasible and high rates of participant satisfaction were also reported.
Broken bridges--new school transitions for students with Autism Spectrum Disorder: A systematic review on difficulties and strategies for success ... Successful transition from primary to secondary school for students with autism spectrum disorder: A systematic literature review. Journal of Research in Childhood Education, 33 (3) (2019), pp ...
Introduction. Research is an important means of finding effective ways to lessen the burden of disease. Most research into autism spectrum disorder (ASD) has been conducted in affluent English-speaking countries, which have extensive professional support services 1.Initial research on autism diagnosis and service delivery in developing countries has suggested that diagnosis can be a cumbersome ...
Effects of physical activity on social, behavioral, and cognitive skills in children and young adults with autism spectrum disorder: a systematic review of the literature. Rev J Autism Dev Disord. 2022;10:749-70. ... Postural control impairments in individuals with autism spectrum disorder: a critical review of current literature.
Autism spectrum disorder (ASD) is a biologically based neurodevelopmental disorder characterized by impairments in two major domains: 1) deficits in social communication and social interaction and 2) restricted repetitive patterns of behavior, interests, and activities. ... A study done to review literature about the use of SSRIs in the ...
Autism Spectrum Disorder and Savant Syndr ome: A Systematic Lit erature Review. Hyun Ok Park. Department of Early Childhood Special Education, Baekseok University, Cheonan, Korea. Objectives: is ...
Autism spectrum disorder has often been assumed to be a protective factor against substance use, yet the extent of substance use in this population has been difficult to determine as limited research has been done on these interacting variables. This systematic literature review examined 26 studies published between 2009 and 2019 to uncover the relationship between autism spectrum disorder and ...
These findings reconcile the discrepant literature and demonstrate that autistic individuals, on average, show a reduced FIE, suggesting that the face processing systems of autistic individuals are less sensitive to face inversion than those of their neurotypical peers. ... Face identity recognition in autism spectrum disorders: a review of ...
last throughout a person's lifetime (1) ASD affects many people, and it has become more. commonly diagnosed in recent years. More boys than. girls receive an ASD diagnosis (2) Autism is a ...
Objective: This review summarizes evidence pertaining to the impact of the COVID-19 pandemic on the psychological health of children and adolescents with autism spectrum disorder (ASD).Materials and Methods: An electronic search was conducted using four major databases: PubMed, ScienceDirect, Web of Science, and Google Scholar.Using an umbrella methodology, the reference lists of relevant ...
The prevalence of autism spectrum disorder (ASD) is approximately one in 68 (Centers for Disease Control and Prevention (CDC, 2014) and almost half (46%) have average through to gifted intelligence (CDC, 2014).In addition, adolescents with ASD have expressed a strong desire to attend post-secondary education (Camarena & Sarigiani, 2009).This goal is increasingly being realized as between 0.7 ...
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by difficulties in social communication and repetitive and stereotyped behaviors. According to the World Health Organization, about 1 in 100 children worldwide has autism.
Abstract. Objective: To review what the literature says about reading abilities of children on the autism spectrum (autism spectrum disorders, ASD) as well as to assess the results of intervention proposals. The broad ASD diagnosis used in the last decades and the resulting changes in the prevalence of these disorders have led to a relevant increase in the number of children diagnosed with ASD ...
Introduction and background. Autism spectrum disorders (ASD) constitute a complex neurodevelopmental disorder influenced by a spectrum of genetic, pathophysiological, and environmental factors, typically manifesting early in childhood and persisting throughout life [].The prevalence of ASD among school-aged children has surged from one in 150 in 2000 to one in 54 in 2016, highlighting its ...
In fact, ubiquitination is intricately linked to autism spectrum disorder (ASD), neurodevelopmental impairments, and intellectual disability (ID) 9. Understanding the UPS's role in these disorders ...
Autism spectrum disorder (ASD) is a prevalent and heterogeneous neurodevelopmental disorder. Risk is attributed to genetic and prenatal environmental factors, though the environmental agents are incompletely characterized. ... The economic costs of autism spectrum disorder: a literature review. J Autism Dev Disord. 2019;49(7):2873-900. https ...
Objective: To review what the literature says about reading abilities of children on the autism spectrum (autism spectrum disorders, ASD) as well as to assess the results of intervention proposals. The broad ASD diagnosis used in the last decades and the resulting changes in the prevalence of these disorders have led to a relevant increase in the number of children diagnosed with ASD in the ...
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by social difficulties, communication difficulties, and repetitive and stereotypical behaviors (Esposito et al., 2023).According to previous studies (Baxter et al., 2015; Zhou, 2020), the global prevalence of ASD is 1-2%, and the prevalence of ASD in China is 0.70%, and this prevalence continues to increase.